Label: LEVOFLOXACIN- levofloxacin solution/ drops
- NDC Code(s): 42571-168-25
- Packager: Micro Labs Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 3, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LEVOFLOXACIN OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for LEVOFLOXACIN OPHTHALMIC SOLUTION.
LEVOFLOXACIN ophthalmic solution 1.5%, sterile topical ophthalmic solution
Initial U.S. Approval: 1996
INDICATIONS AND USAGE
Levofloxacin ophthalmic solution is a topical quinolone anti-microbial indicated for the treatment of corneal ulcer caused by susceptible strains of the following bacteria:
Corynebacterium species *
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus pneumonia
Viridans group streptococci*
Pseudomonas aeruginosa
Serratia marcescens*
*Efficacy for this organism was studied in fewer than 10 infections. (1)
DOSAGE AND ADMINISTRATION
Days 1 through 3:
Instill one to two drops in the affected eye(s) every 30 minutes to 2 hours while awake and approximately 4 and 6 hours after retiring.
Day 4 through treatment completion:
Instill one to two drops in the affected eye(s) every 1 to 4 hours while awake. (2)
DOSAGE FORMS AND STRENGTHS
5 cc container filled with 5 mL sterile ophthalmic solution of levofloxacin, 1.5% (3)
CONTRAINDICATIONS
Levofloxacin ophthalmic solution is contraindicated in patients with a history of hypersensitivity to levofloxacin, to other quinolones, or to any of the components in this medication. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most frequently reported adverse reactions in the overall study population were headache and a taste disturbance following instillation. These reactions occurred in approximately 8 to 10% of patients. (6)
To report SUSPECTED ADVERSE REACTIONS,contact Micro Labs USA, Inc. at 1-855-839-8195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Growth of Resistant Organisms with Prolonged Use
5.3 Avoidance of Contact Lens Wear
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Avoid Contamination of the Product
17.2 Avoid Contact Lens Wear
17.3 Hypersensitivity Reactions
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Levofloxacin ophthalmic solution is indicated for the treatment of corneal ulcer caused by susceptible strains of the following bacteria:
Gram-positive bacteria:
Corynebacterium species
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus pneumonia
Viridans group streptococci*
Gram-negative bacteria:
Pseudomonas aeruginosa
Serratia marcescens*
*Efficacy for this organism was studied in fewer than 10 infections
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
In patients receiving systemically administered quinolones, including levofloxacin, serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported, some following the first dose. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema, (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria and itching. If an allergic reaction to levofloxacin occurs, discontinue the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management should be administered as clinically indicated.
5.2 Growth of Resistant Organisms with Prolonged Use
As with other anti-infectives, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and where appropriate, fluorescein staining.
-
6 ADVERSE REACTIONS
The most frequently reported adverse reactions in the overall study population were headache and a taste disturbance following instillation. These reactions occurred in approximately 8 to 10% of patients. Adverse reactions occurring in approximately 1 to 2% of patients included decreased/blurred vision, diarrhea, dyspepsia, fever, infection, instillation site irritation/discomfort, ocular infection, nausea, ocular pain/discomfort, and throat irritation. Other reported ocular reactions occurring in less than 1% of patients included chemosis, corneal erosion, diplopia, floaters, hyperemia, lid edema, and lid erythema.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Teratogenic Effects: Levofloxacin at oral doses of 810 mg/kg/day in rats caused decreased fetal body weight and increased fetal mortality. No teratogenic effect was observed when rabbits were dosed orally as high as 50 mg/kg/day, at which systemic exposure was estimated to be 250 times that observed at the maximum recommended human ophthalmic dose, or when dosed intravenously as high as 25 mg/kg/day, at which systemic exposure was estimated to be 120 times that observed at the maximum recommended human ophthalmic dose.
There are, however, no adequate and well-controlled studies in pregnant women. Levofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Levofloxacin has not been measured in human milk. Based on data from ofloxacin, it can be presumed that levofloxacin is excreted in human milk. Caution should be exercised when levofloxacin is administered to a nursing mother.
8.4 Pediatric Use
Safety and effectiveness in children below the age of six years have not been established. Oral administration of systemic quinolones has been shown to cause arthropathy in immature animals. There is no evidence that the ophthalmic administration of levofloxacin has any effect on weight bearing joints.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Levofloxacin ophthalmic solution 1.5% is a sterile topical ophthalmic solution. Levofloxacin is a fluoroquinolone antibacterial active against a broad spectrum of Gram-positive and Gram-negative ocular pathogens. Levofloxacin is a fluorinated 4-quinolone containing a six-member (pyridobenzoxazine) ring from positions 1 to 8 of the basic ring structure. Levofloxacin is the pure (-)-( S)-enantiomer of the racemic drug substance, ofloxacin. It is more soluble in water at neutral pH than ofloxacin.
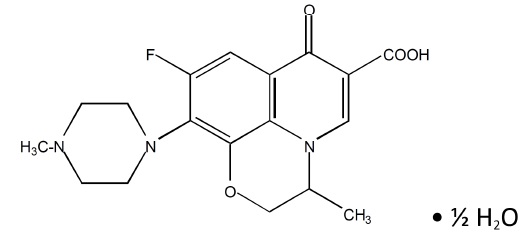
C 18H 20FN 3O 4 ·½ H2O Mol Wt 370.38
Chemical Name: (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7 H- pyrido[1,2,3- de]-1,4 benzoxazine-6-carboxylic acid hemihydrate. Levofloxacin (hemihydrate) USP is a yellowish-white crystalline powder. Each mL of levofloxacin ophthalmic solution contains 15.36 mg of levofloxacin hemihydrate USP equivalent to 15 mg levofloxacin.
Contains: Active: Levofloxacin (hemihydrate) USP is a light yellowish-white; Inactives: glycerin and water for injection. May also contain hydrochloric acid and/or sodium hydroxide to adjust pH to approximately 6.7. Levofloxacin ophthalmic solution is isotonic with an osmolality of approximately 291 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Levofloxacin is a member of the fluoroquinolone class of anti-microbial drug (See 12.4 Microbiology) .
12.3 Pharmacokinetics
Levofloxacin concentration in plasma was measured in 14 healthy adult volunteers during a 16-day course of treatment with levofloxacin ophthalmic solution. The dosing schedule was 1 to 2 drops per eye once in the morning on Days 1 and 16; 1 to 2 drops per eye every two hours Days 2 through 8; and 1 to 2 per eye every four hours Days 9 through 15. The mean levofloxacin concentration in plasma 1 hour post dose ranged from 3.13 ng/mL on Day 1 to 10.4 ng/mL on Day 16.
Maximum mean levofloxacin concentrations increased from 3.22 ng/mL on Day 1 to 10.9 ng/mL on Day 16, which is more than 400 times lower than those reported after standard oral doses of levofloxacin.
Levofloxacin concentration in tears was measured in 100 healthy adult volunteers at various time points following instillation of 2 drops of levofloxacin ophthalmic solution. Mean tear concentration measured 15 minutes after instillation was 757 mcg/mL.
12.4 Microbiology
Levofloxacin is the L-isomer of the racemate, ofloxacin, a quinolone antimicrobial agent. The antibacterial activity of ofloxacin resides primarily in the L-isomer. The mechanism of action of levofloxacin and other fluoroquinolone antimicrobials involves the inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair, and recombination.
Levofloxacin has in vitro activity against a wide range of Gram-negative and Gram-positive microorganisms and is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Fluoroquinolones, including levofloxacin, differ in chemical structure and mode of action from ß-lactam antibiotics and aminoglycosides, and therefore may be active against bacteria resistant to ß-lactam antibiotics and aminoglycosides. Additionally, ß-lactam antibiotics and aminoglycosides may be active against bacteria resistant to levofloxacin. Resistance to levofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10 - 9 to 10 - 10).
Levofloxacin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-positive microorganisms:
Corynebacterium species*
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus pneumoniae
Viridans group streptococci*
Aerobic gram-negative microorganisms:
Pseudomonas aeruginosa
Serratia marcescens*
*Efficacy for this organism was studied in fewer than 10 infections.
The following in vitro data are also available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of levofloxacin in treating ophthalmological infections due to these microorganisms have not been established in adequate and well controlled trials.
These organisms are considered susceptible when evaluated using systemic breakpoints. However, a correlation between the in vitro systemic breakpoint and ophthalmological efficacy has not been established. The list of organisms is provided as guidance only in assessing the potential treatment of corneal ulcer. Levofloxacin exhibits in vitro minimal inhibitory concentrations (MICs) of 2 mcg/mL or less (systemic susceptible breakpoint) against most (≥ 90%) strains of the following ocular pathogens:
Aerobic gram-positive microorganisms:
Enterococcus faecalis (many strains are only moderately susceptible)
Staphylococcus saprophyticus
Streptococcus agalactiae
Streptococcus pyogenes
Streptococcus (Group C/F)
Streptococcus (Group G)
Aerobic gram-negative microorganisms:
Acinetobacter baumannii
Acinetobacter lwoffii
Citrobacter koseri
Citrobacter freundii
Enterobacter aerogenes
Enterobacter cloacae
Escherichia coli
Haemophilus influenzae
Haemophilus parainfluenzae
Klebsiella oxytoca
Klebsiella pneumonia
Legionella pneumophila
Moraxella catarrhalis
Morganella morganii
Neisseria gonorrhoeae
Pantoea agglomerans
Proteus mirabilis
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Pseudomonas fluorescens
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a long term carcinogenicity study in rats, levofloxacin exhibited no carcinogenic or tumorigenic potential following daily dietary administration for 2 years at doses up to 100 mg/kg/day, corresponding to plasma levels that were 245 times maximum clinical exposure, based on C max.
Levofloxacin was not mutagenic in the following assays: Ames bacterial mutation assay (S. typhimurium and E. coli) CHO/HGPRT forward mutation assay, mouse micronucleus test, mouse dominant lethal test, rat unscheduled DNA synthesis assay, and the in vivo mouse sister chromatid exchange assay. It was positive in the in vitro chromosomal aberration (CHL cell line) and in vitro sister chromatid exchange (CHL/IU cell line) assays. Levofloxacin caused no impairment of fertility or reproduction in rats at oral doses as high as 360 mg/kg/day, at which systemic exposure was estimated to be 2,600 times that at the maximum recommended human ophthalmic dose.
-
14 CLINICAL STUDIES
In two randomized, double-masked, multi-center, controlled clinical trials of 280 patients with positive cultures, subjects were dosed with levofloxacin or ofloxacin 0.3% ophthalmic solution.
Dosing occurred on Days 1 through 3 every two hours while awake and 4 and 6 hours after retiring. Dosing occurred on Day 4 through treatment completion 4 times daily while awake. Clinical cure was defined as complete re-epithelialization and no progression of the infiltrate for two consecutive visits. The levofloxacin treated subjects had an approximately equal mean clinical cure rate of 80% (73% to 87%) compared to 84% (82% to 86%) for the subjects treated with ofloxacin 0.3% ophthalmic solution.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Levofloxacin ophthalmic solution 1.5% is supplied in a sterile with a white opaque cylindrical shaped low density polyethylene bottle with an open white opaque cone shaped low density polyethylene controlled dropper tip and a tan color cone shaped high density polyethylene cap in the following size:
5 mL fill in 5 cc container– NDC 42571-168-25
Storage: Store at 15° to 25°C (59° to 77°F).
-
17 PATIENT COUNSELING INFORMATION
17.1 Avoid Contamination of the Product
Advise patients to avoid contaminating the applicator tip with material from the eye, finger, or other source.
17.2 Avoid Contact Lens Wear
Advise patients not to wear contact lenses if they have signs and symptoms of corneal ulcer.
17.3 Hypersensitivity Reactions
Systemically administered quinolones, including levofloxacin, have been associated with hypersensitivity reactions, even following a single dose. Advise patients to discontinue use immediately and contact their physician at the first sign of a rash or allergic reactions.
-
INSTRUCTIONS FOR USE
Before you use Levofloxacin Ophthalmic Solution for the first time:
1. Check to make sure that the tamper evident ring between the bottle and the cap is not broken ( See Figure A). If the tamper evident ring is broken or missing, contact your pharmacist.
2. Tear off the tamper evident ring ( See Figure B).
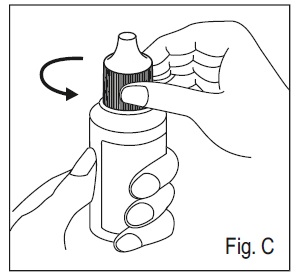
3. To open the bottle, remove the cap by turning it in the counterclockwise direction ( See Figure C).
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Micro Labs Limited
Banglore-560099. INDIA.
Manufactured for:
Micro Labs USA Inc.
Basking Ridge, NJ 07920 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEVOFLOXACIN
levofloxacin solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42571-168 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOFLOXACIN (UNII: 6GNT3Y5LMF) (LEVOFLOXACIN ANHYDROUS - UNII:RIX4E89Y14) LEVOFLOXACIN ANHYDROUS 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (greenish yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42571-168-25 1 in 1 CARTON 04/16/2019 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205600 04/16/2019 Labeler - Micro Labs Limited (862174955) Establishment Name Address ID/FEI Business Operations Micro Labs Limited 677600482 manufacture(42571-168)




