ACTIVE FE- .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, iron pentacarbonyl, magnesium oxide, zinc oxide, and cupric oxide tablet
GM Pharmaceuticals, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
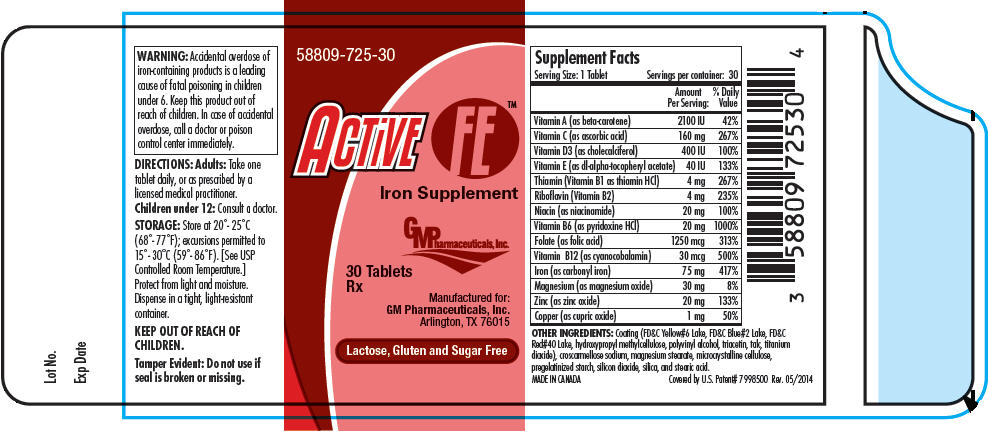
Active FE™
Iron Supplement
Rx
| Supplement Facts
Serving Size: 1 Tablet Servings per container: 30 |
||
|---|---|---|
| Amount Per Serving: | % Daily Value | |
| Vitamin A (as beta-carotene) | 2100 IU | 42% |
| Vitamin C (as ascorbic acid) | 160 mg | 267% |
| Vitamin D3 (as cholecalciferol) | 400 IU | 100% |
| Vitamin E (as dl-alpha-tocopheryl acetate) | 40 IU | 133% |
| Thiamin (Vitamin B1 as thiamin HCl) | 4 mg | 267% |
| Riboflavin (Vitamin B2) | 4 mg | 235% |
| Niacin (as niacinamide) | 20 mg | 100% |
| Vitamin B6 (as pyridoxine HCl) | 20 mg | 1000% |
| Folate (as folic acid) | 1250 mcg | 313% |
| Vitamin B12 (as cyanocobalamin) | 30 mcg | 500% |
| Iron (as carbonyl iron) | 75 mg | 417% |
| Magnesium (as magnesium oxide) | 30 mg | 8% |
| Zinc (as zinc oxide) | 20 mg | 133% |
| Copper (as cupric oxide) | 1 mg | 50% |
OTHER INGREDIENTS: Coating (FD&C Yellow#6 Lake, FD&C Blue#2 Lake, FD&C Red#40 Lake, hydroxypropyl methylcellulose, polyvinyl alcohol, triacetin, talc, titanium dioxide), croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch, silicon dioxide, silica, and stearic acid.
CLINICAL PHARMACOLOGY
Iron is an essential component in the formation of hemoglobin. Adequate amounts of iron are necessary for effective erythropoiesis. Iron also serves as a cofactor of several essential enzymes, including cytochromes that are involved in electron transport. Folic acid is required for nucleoprotein synthesis and the maintenance of normal erythropoiesis. Folic acid is converted in the liver and plasma to its metabolically active form, tetrahydrofolic acid, by dihydrofolate reductase. Vitamin B 12 is required for the maintenance of normal erythropoiesis, nucleoprotein and myelin synthesis, cell reproduction and normal growth. Intrinsic factor, a glycoprotein secreted by the gastric mucosa, is required for active absorption of vitamin B 12 from the gastrointestinal tract.
INDICATIONS AND USAGE
ACTIVE FE™ is indicated for the prevention and treatment of iron deficiency anemia and/or nutritional megaloblastic anemias. 1
- 1
- This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent disease.
CONTRAINDICATIONS
ACTIVE FE™ is contraindicated in patients with a known hypersensitivity to any of the components of this product. Hemolytic anemia, hemochromatosis and hemosiderosis are contraindications to iron therapy.
WARNINGS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B 12 is deficient.
PRECAUTIONS
General
Do not exceed recommended dose. The type of anemia and the underlying cause or causes should be determined before starting therapy with ACTIVE FE™. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
ADVERSE REACTIONS
Adverse reactions with iron therapy may include GI irritations, constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Adverse reactions with iron therapy are usually transient. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Carbonyl iron based products may decrease the absorption of medicines. Talk to your doctor and pharmacist before taking carbonyl products if you take any prescription or over-the-counter medicines.
OVERDOSAGE
The clinical course of acute iron overdosage can be variable. Initial symptoms may include abdominal pain, nausea, vomiting, diarrhea, tarry stools melena, hematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock and coma.
HOW SUPPLIED
ACTIVE FE™ is supplied as red capsule shaped tablets with imprint FE1 in child-resistant bottles containing 30 tablets. (58809-725-30)
| ACTIVE FE
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, iron pentacarbonyl, magnesium oxide, zinc oxide, and cupric oxide tablet |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - GM Pharmaceuticals, Inc. (793000860) |