CASTELLANI PAINT- phenol liquid
Pedinol Pharmacal, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
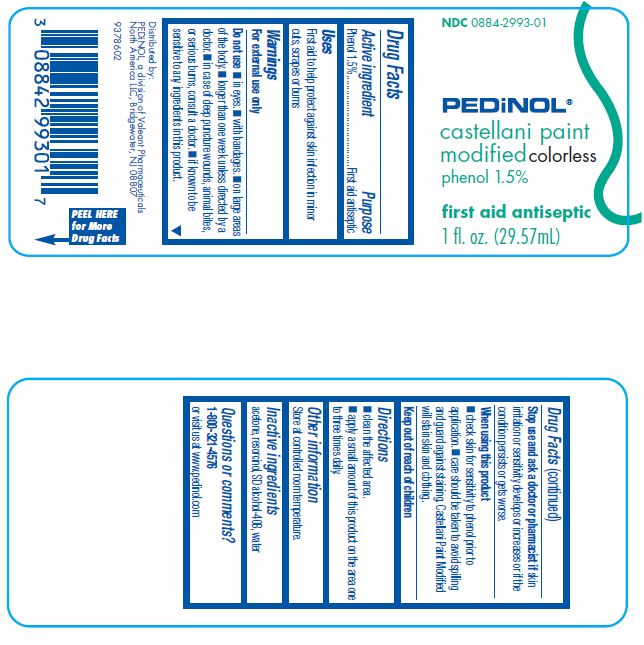
Castellani Paint Modified (colorless)
Warnings
For external use only
Do not use
- •
- in eyes.
- •
- with bandages.
- •
- on large areas of the body.
- •
- longer than one week unless directed by a doctor.
- •
- in case of deep puncture wounds, animal bites, or serious burns, consult a doctor.
- •
- if known to be sensitive to any ingredients in this product.
Stop use and ask a doctor or pharmacist if skin irritation or sensitivity develops or increases or if the condition persists or gets worse.
| CASTELLANI PAINT
phenol liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Pedinol Pharmacal, Inc. (064737125) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cospro Development Corp | 785638821 | MANUFACTURE(0884-2993) | |
Revised: 12/2018
Document Id: c8b87ffd-8976-4c99-b844-e938af41df53
Set id: bf6845e5-16f3-4828-bfbf-2bc2596a02f3
Version: 4
Effective Time: 20181203
Pedinol Pharmacal, Inc.