MENS ROGAINE EXTRA STRENGTH- minoxidil solution
Johnson & Johnson Consumer Inc.
----------
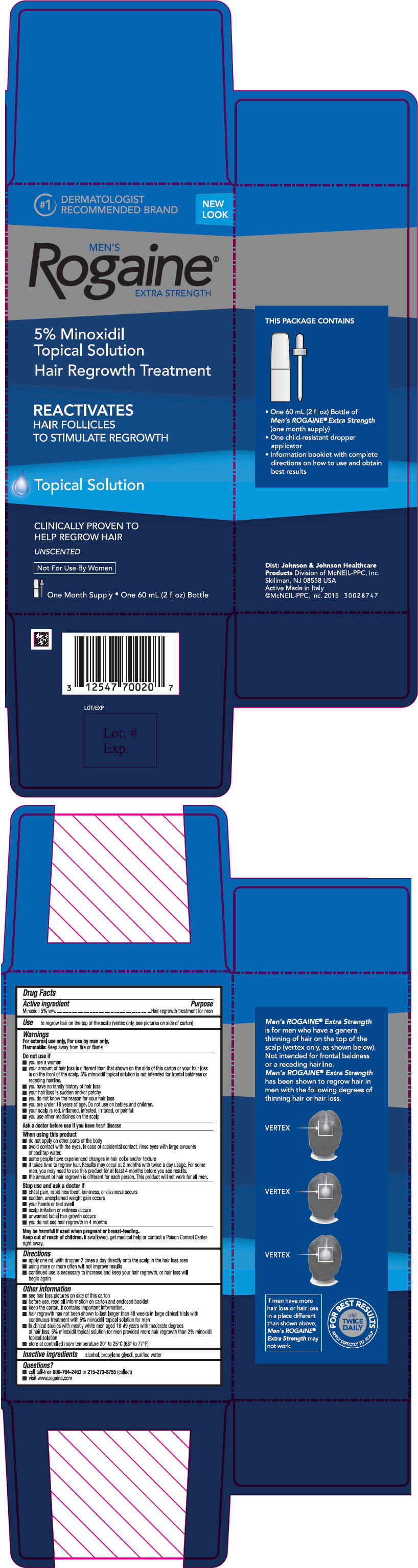
Men's Rogaine Extra Strength 5%
Unscented Solution
Carton
Warnings
For external use only. For use by men only.
Flammable: Keep away from fire or flame
Do not use if
- you are a woman
- your amount of hair loss is different than that shown on the side of this carton or your hair loss is on the front of the scalp. 5% minoxidil topical solution is not intended for frontal baldness or receding hairline.
- you have no family history of hair loss
- your hair loss is sudden and/or patchy
- you do not know the reason for your hair loss
- you are under 18 years of age. Do not use on babies and children.
- your scalp is red, inflamed, infected, irritated, or painful
- you use other medicines on the scalp
When using this product
- do not apply on other parts of the body
- avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
- some people have experienced changes in hair color and/or texture
- it takes time to regrow hair. Results may occur at 2 months with twice a day usage. For some men, you may need to use this product for at least 4 months before you see results.
- the amount of hair regrowth is different for each person. This product will not work for all men.
Directions
- apply one mL with dropper 2 times a day directly onto the scalp in the hair loss area
- using more or more often will not improve results
- continued use is necessary to increase and keep your hair regrowth, or hair loss will begin again
Other information
- see hair loss pictures on side of this carton
- before use, read all information on carton and enclosed booklet
- keep the carton. It contains important information.
- hair regrowth has not been shown to last longer than 48 weeks in large clinical trials with continuous treatment with 5% minoxidil topical solution for men
- in clinical studies with mostly white men aged 18-49 years with moderate degrees of hair loss, 5% minoxidil topical solution for men provided more hair regrowth than 2% minoxidil topical solution
- store at controlled room temperature 20° to 25° C (68° to 77° F)
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Carton
#1 DERMATOLOGIST
RECOMMENDED BRAND
NEW LOOK
MEN'S
Rogaine®
EXTRA STRENGTH
5% Minoxidil
Topical Solution
Hair Regrowth Treatment
REACTIVATES
HAIR FOLLICLES
TO STIMULATE REGROWTH
Topical Solution
CLINICALLY PROVEN TO
HELP REGROW HAIR
UNSCENTED
Not For Use By Women
One Month Supply * One 60 mL (2 fl oz) Bottle
| MENS ROGAINE
EXTRA STRENGTH
minoxidil solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 12/2016
Document Id: a3d4f3d0-402d-48f2-9f2d-578dc79e0ba2
Set id: beb0eb05-1ffd-4b68-bc61-35805e2bebdd
Version: 4
Effective Time: 20161220
Johnson & Johnson Consumer Inc.