Label: ACL10SPS SINGLE SHOT EPIDURAL- kit
- NHRIC Code(s): 55553-432-02
- NDC Code(s): 52380-0001-3
- Packager: Clint Pharmaceuticals, Inc.
- Category: MEDICAL DEVICE
Drug Label Information
Updated June 25, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
WARNINGS
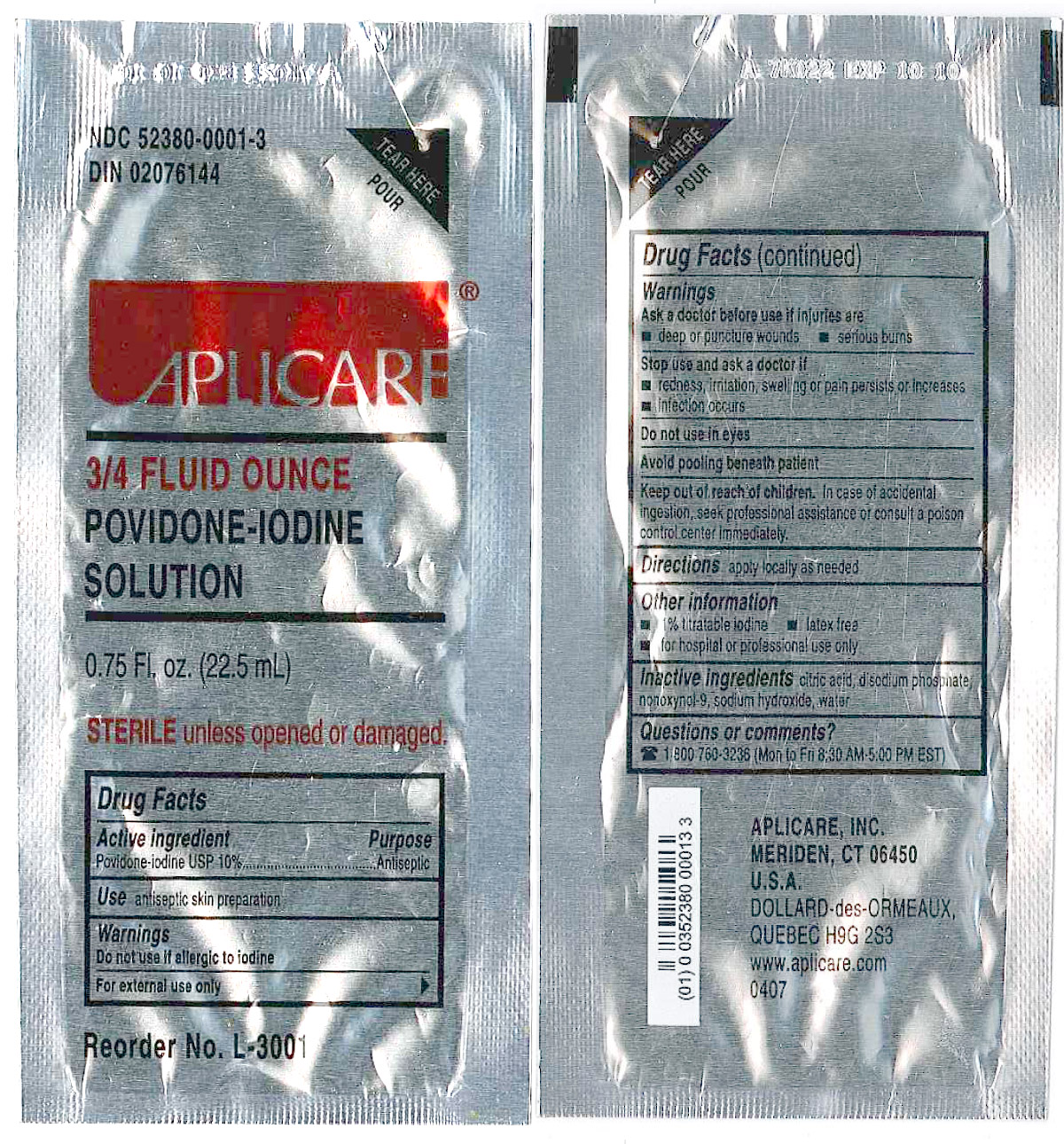
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children. In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACL10SPS SINGLE SHOT EPIDURAL
regional anesthesia kit kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:55553-432 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:55553-432-02 30 in 1 CASE 1 1 in 1 PACKAGE, COMBINATION Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 22.5 mL Part 1 of 1 APLICARE POVIDONE-IODINE
povidone-iodine solutionProduct Information Item Code (Source) NDC:52380-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52380-0001-3 22.5 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/01/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K965017 01/21/2009 Labeler - Clint Pharmaceuticals, Inc. (609197785) Registrant - Smiths Medical ASD, Inc. (137835299) Establishment Name Address ID/FEI Business Operations Smiths Medical ASD, Inc. 137835299 relabel, manufacture Establishment Name Address ID/FEI Business Operations Aplicare, Inc. 107255002 manufacture