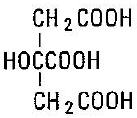RENACIDIN- citric acid, gluconolactone and magnesium carbonate solution
United-Guardian, Inc.
----------
Renacidin® (Citric Acid, Glucono Delta-Lactone, and Magnesium Carbonate) Irrigation
DESCRIPTION
Renacidin® (Citric Acid, Glucono delta-lactone, and Magnesium Carbonate) Irrigation is a sterile, non-pyrogenic irrigation for use within the urinary tract in the prevention and dissolution of calculi.
Each 100 mL of Renacidin Irrigation contains:
Active ingredients:
Citric Acid (anhydrous), USP 6.602 grams
C6H8O7
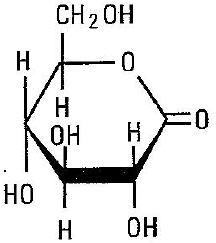
Glucono delta-lactone, USP 0.198 grams
C6H10O6
Magnesium Carbonate, USP 3.376 grams
(MgCO3)4 · Mg(OH)2 · 5H2O
Citric Acid
Glucono delta-lactone
Magnesium Carbonate
(MgCO3)4 · Mg(OH)2 · 5H2O
Inert ingredients:
Benzoic Acid, USP 0.023 grams
Solution pH: 3.85 (3.5-4.2)
CLINICAL PHARMACOLOGY
Renacidin Irrigation's action on susceptible apatite calculi results from an exchange of magnesium from the irrigating solution for calcium contained in the stone matrix. The magnesium salts thereby formed are soluble in the gluconocitrate irrigating solution resulting in the dissolution of the calculus. Struvite calculi are composed mainly of magnesium ammonium phosphates which are solubilized by Renacidin Irrigation due to its acidic pH.
Renacidin Irrigation is not effective for dissolution of calcium oxalate, uric acid or cysteine stones.
INDICATIONS AND USAGE
Renacidin Irrigation is indicated for use by local irrigation in the dissolution of renal calculi composed of apatite (a calcium carbonate phosphate compound) or struvite (magnesium ammonium phosphates in patients who are not candidates for surgical removal of the calculi.
It may also be used as adjunctive therapy to dissolve residual apatite or struvite calculi and fragments after surgery or to achieve partial dissolution of renal calculi to facilitate surgical removal.
Renacidin Irrigation is also indicated for dissolution of bladder calculi of the struvite or apatite variety by local intermittent irrigation through a urethral catheter or cystostomy catheter as an alternative or adjunct to surgical procedures.
Renacidin Irrigation is also indicated for use as an intermittent irritating solution to prevent or minimize encrustations of indwelling urinary tract catheters.
Since many complications are experienced by patients receiving infusions of Renacidin Irrigation into the renal pelvis, considerable caution must be employed. Additionally, hospitalization is prolonged for days to weeks when chemolytic therapy is used in lieu of, or following surgery. For these reasons, use of this therapy should be reserved for selected patients.
Renacidin Irrigation is not indicated for dissolution of calcium oxalate, uric acid or cysteine calculi.
CONTRAINDICATIONS
The use of Renacidin Irrigation in the treatment of renal calculi is contraindicated in patients with urinary tract infections. Urea splitting bacteria reside within struvite and apatite stones which therefore serve as a source of infection. Dissolution therapy with Renacidin Irrigation in the presence of an infected urinary tract may lead to sepsis and death. Urine specimens should be obtained for culture prior to initiating chemolytic therapy of the renal pelvis. Appropriate antibiotic therapy should be instituted to treat any infection detected. A sterile urine must be present prior to initiating therapy. An infected stone can serve as a continual source for infection and, therefore, antibiotic therapy should be continued throughout the course of dissolution therapy.
Renacidin Irrigation is contraindicated in the presence of demonstrable urinary tract extravasation.
WARNINGS
Renacidin Irrigation use should be stopped immediately if the patient develops fever, urinary tract infection, signs and symptoms consistent with urinary tract infection, or persistent flank pain. Irrigation should be stopped if hypermagnesemia or elevated serum creatinine develops.
Severe hypermagnesemia has been reported with Renacidin Irrigation. Caution should be employed when irrigating the renal pelvis of patients with impaired renal function. Patients should be observed for early signs and symptoms of hypermagnesemia including nausea, lethargy, confusion and hypotension. Severe hypermagnesemia may result in hyporeflexia, dyspnea, apnea, coma, cardiac arrest and subsequent death. Serum magnesium levels should be monitored and deep tendon reflexes should be evaluated. Treatment of hypermagnesemia should include discontinuation of Renacidin Irrigation followed by medical therapy with intravenous calcium gluconate, fluids and diuresis in severe cases.
PRECAUTIONS
Care must be taken during chemolysis of renal calculi with Renacidin Irrigation to maintain the patency of the irrigating catheter. Calculus fragments and debris may obstruct the outflow catheter. Continued irrigation under those circumstances leads to increased intrapelvic pressure with a danger of tissue damage or absorption of the irrigating solution. Catheter outflow blockage may be prevented by flushing the catheter with saline and repositioning of the catheter. Frequent monitoring of the system should be performed by a nurse, an aide or any person with sufficient skills to be able to detect any problems with the patency of the catheter. At the first sign of obstruction, irrigation should be discontinued and the system disconnected.
Intrapelvic pressures must be maintained at or below 25 cm of water. The preferred method of pressure control is the insertion of an open Y connection pop-off valve into the infusion line allowing immediate decompression if pressure exceeds 25 cm of water. An alternative method has been proposed to direct or stop the flow of the irrigating solution to prevent increased intrapelvic pressure: placement of a pinch clamp on the inflow line which can be used by the patient or nurse to stop the irrigation at the first sign of flank pain. However, extreme caution must be taken when relying on cooperation of the patient. Patients may not be sufficiently alert to detect signs and symptoms of out-flow obstruction. This is especially true in elderly patients or patients who have been sedated or who have severe neurological dysfunction with varying degrees of sensory loss and/or motor paralysis.
Patients with indwelling urethral or cystostomy catheters frequently have vesicoureteral reflux. Cystogram prior to initiation of Renacidin Irrigation is essential for such patients. If reflux is demonstrated, all precautions recommended for renal pelvis irrigation must be taken.
Throughout the course of therapy, patients should be monitored to assure safety. Serum creatinine, phosphate and magnesium should be obtained every several days. Urine specimens should be collected for culture and antibacterial sensitivity every three days or less and at the first sign of fever. The irrigation should be stopped if any culture exhibits growth and appropriate antibacterial therapy should be initiated. The irrigation may be started again after a course of antibacterial therapy upon demonstration of a sterile urine. Struvite calculi frequently contain bacteria within the stone and antibacterial therapy should therefore be continued throughout the course of dissolution therapy. Hypermagnesemia or an elevated serum creatinine level are indications to halt the irrigation until they return to pre-irrigation levels. Evidence of severe urothelial edema on X-ray is also an indication for temporarily halting the irrigation until the complication resolves.
Concurrent use of magnesium containing medications may contribute to production of hypermagnesemia and is not recommended.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies to evaluate carcinogenic potential of Renacidin Irrigation in animals have not been conducted. Mutagenicity studies have not been conducted.
Pregnancy Category C
Animal reproduction studies have not been conducted with Renacidin Irrigation. It is also not known whether Renacidin Irrigation can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Renacidin Irrigation should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
The most common adverse reaction in selected case series is transient flank pain which occurs in most patients. Additional common reactions include urothelial ulceration and/or edema (13%) or fever (20% but up to 40% in some case series). Other adverse reactions which occur in 1-10% of cases include: urinary tract infection, back pain, dysuria, transient hematuria, nausea, hypermagnesemia, hyperphosphatemia, elevated serum creatinine, candidiasis, and bladder irritability. Adverse reactions which occur in less than 1 % of patients include: septicemia, ileus, vomiting and thrombophlebitis. Death from sepsis has been reported.
DOSAGE AND ADMINISTRATION
Renacidin Irrigation (sterile, non-pyrogenic) in water for local irrigation within the urinary tract.
The action of Renacidin Irrigation in the prevention and dissolution of calculi results from an ion exchange mechanism or solvent action. (See Clinical Pharmacology)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Renal Calculi: See Precautions. It is essential that patients be free from urinary tract infections prior to initiating chemolytic therapy. Urine specimens should be collected for culture and appropriate antibiotic therapy should be initiated for any bacteria identified. A nephrostomy tube is placed at surgery or percutaneously to permit lavage of the calculi. A single catheter may be sufficient if the calculus is not obstructing the ureter or ureteropelvic junction. In patients with an obstructed ureter, a retrograde catheter can be placed through the ureter to the renal pelvis via a cystoscope. This second catheter is used to irrigate the calculus while the percutaneous nephrostomy tube is used for drainage.
Plain radiographs and nephrostomograms are performed to assure proper placement of the catheter(s). Pressure measurements are made under fluoroscopy to assure that 2-3 mL/min can be infused without causing pain, pyelovenous or pyelotubular backflow or manometric evidence of elevated pressure within the collecting system.
For postoperative patients irrigation should not be started before the fourth or fifth postoperative day. Irrigation of the renal pelvis is begun with sterile saline only after a sterile urine has been demonstrated. The saline is infused at a rate of 60 mL/hr initially and the rate is increased until pain or an elevated pressure (25 cm H20) appears, or until a maximum flow-rate of 120 mL/hr is achieved. The site of insertion should be inspected for leakage. If leakage occurs, the irrigation is discontinued temporarily to allow for complete healing around the nephrostomy tube.
If no leakage or flank pain occur, irrigation is then started with Renacidin Irrigation with a flow rate equal to the maximum rate achieved with the saline solution. A clamp should be placed on the inflow tube and patients (see Precautions) and nursing personnel should be instructed to stop the irrigating solution whenever pain develops. Nursing personnel who are responsible for performing the irrigation must be instructed concerning the location of the nephrostomy tube(s) and the direction of flow of the irrigating solution to insure against misconnection of the inflowing and egress tubes. Nephrostomograms should be performed periodically to assure proper placement of the catheter tip and to assess efficacy. If stones fail. to change size after several days of adequate irrigation the procedure should be discontinued.
Upon demonstration of complete dissolution of the calculus the inflow tube is clamped and left in place for a few days to ensure that no obstruction exists, after which time the nephrostomy tube is removed.
Bladder Calculi: Chemolysis of bladder calculi is used as an alternative to cystoscopic or surgical removal of the stones in patients who refuse surgery or cystoscopic removal or in whom these procedures constitute an unwarranted risk. Following appropriate studies to evaluate possible vesicoureteral reflux, thirty mL of Renacidin Irrigation is instilled through a urinary catheter into the bladder and the catheter is clamped for 30-60 minutes. The clamp is then released and the bladder is drained. This is repeated 4-6 times a day. A continuous drip through a 3-way Foley catheter is an alternative means of dissolving bladder stones. In the presence of bladder spasm and associated high pressure reflux, all precautions required for irrigation of the renal pelvis must be observed.
Indwelling Urinary Tract Catheter Encrustation: Periodic instillation of Renacidin Irrigation is indicated to minimize or prevent encrustation of indwelling catheters which frequently results in plugging of the catheter and discomfort to the patient. This is accomplished by instilling 30 mL of the solution through the catheter and then clamping the catheter for 10 minutes, after which the clamp is removed to allow drainage of the bladder. This process is repeated 3 times a day.
HOW SUPPLIED
Renacidin Irrigation is available as a sterile, non-pyrogenic solution in 500 mL containers. Exposure of Renacidin Irrigation to heat or cold should be minimized. Renacidin Irrigation should be stored at controlled room temperature, 59° to 86°F (15° to 30°C). Avoid excessive heat or cold (keep from freezing). Brief exposure to temperatures of up to 40°C or temperatures down to 5°C does not adversely affect the product.
NDC: 0327-0011-05
Product Code: RN500
Revised: November, 2006
Jointly manufactured by
GUARDIAN LABORATORIES
a division of
UNITED-GUARDIAN, INC.
Hauppauge, N.Y. 11788
and HOSPIRA, INC.,
LAKE FOREST, IL 60045
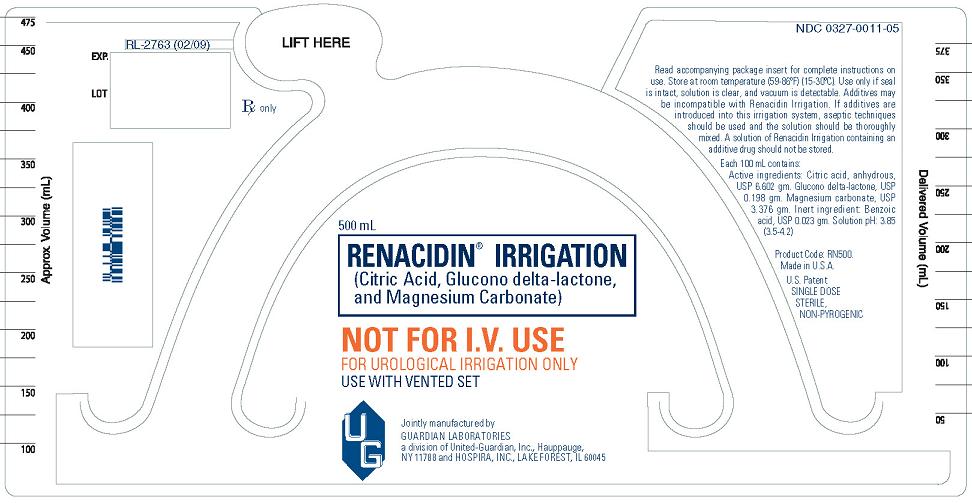
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NOT FOR I.V. USE
FOR UROLOGICAL IRRIGATION ONLY
USE WITH VENTED SET
Read accompanying package insert for complete instructions on use. Store at room temperature (59°-86°F) (15°-30°C). Use only if seal is intact, solution is clear, and vacuum is detectable. Additives may be incompatible with Renacidin Irrigation. If additives are introduced into this irrigation system, aseptic techniques should be used and the solution should be thoroughly mixed. A solution of Renacidin Irrigation containing an additive drug should not be stored.
Each 100 mL contains:
Active ingredients: Citric acid, anhydrous, USP 6.602 gm. Glucono delta-lactone, USP 0.198 gm. Magnesium carbonate, USP 3.376 gm. Inert ingredient: Benzoic acid, USP 0.023 gm. Solution pH: 3.85 (3.5-4.2)
Product Code: RN500.
Made in U.S.A.
U.S. Patent
SINGLE DOSE STERILE, NON-PYROGENIC
Bottle Label
| RENACIDIN
citric acid, gluconolactone and magnesium carbonate solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - United-Guardian, Inc. (050594555) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| United-Guardian, Inc. | 050594555 | MANUFACTURE(0327-0011) | |