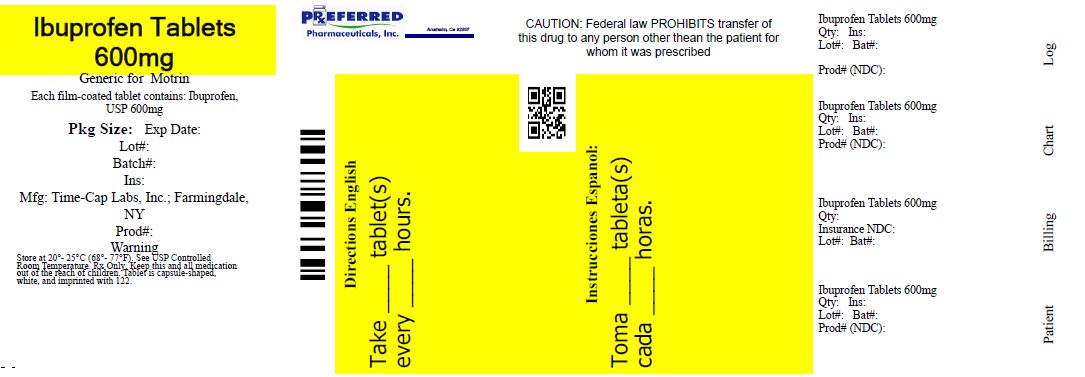
IBUPROFEN- ibuprofen tablet, film coated
Preferred Pharmaceuticals Inc.
----------
IBUPROFEN 400 MG - 600 MG AND 800 MG TABLETS
| IBUPROFEN
ibuprofen tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | REPACK(68788-7602) | |
Revised: 7/2023
Document Id: d83ca34e-c14d-41b0-a101-2d9fa4526b01
Set id: bd7d9986-f0ec-4013-8b57-6ce7cc013cf6
Version: 4
Effective Time: 20230713
Preferred Pharmaceuticals Inc.