Label: MENSTRUAL RELIEF- acetaminophen, caffeine and pyrilamine maleate tablet
- NDC Code(s): 36800-187-24
- Packager: TOP CARE (Topco Associates LLC)
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 19, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
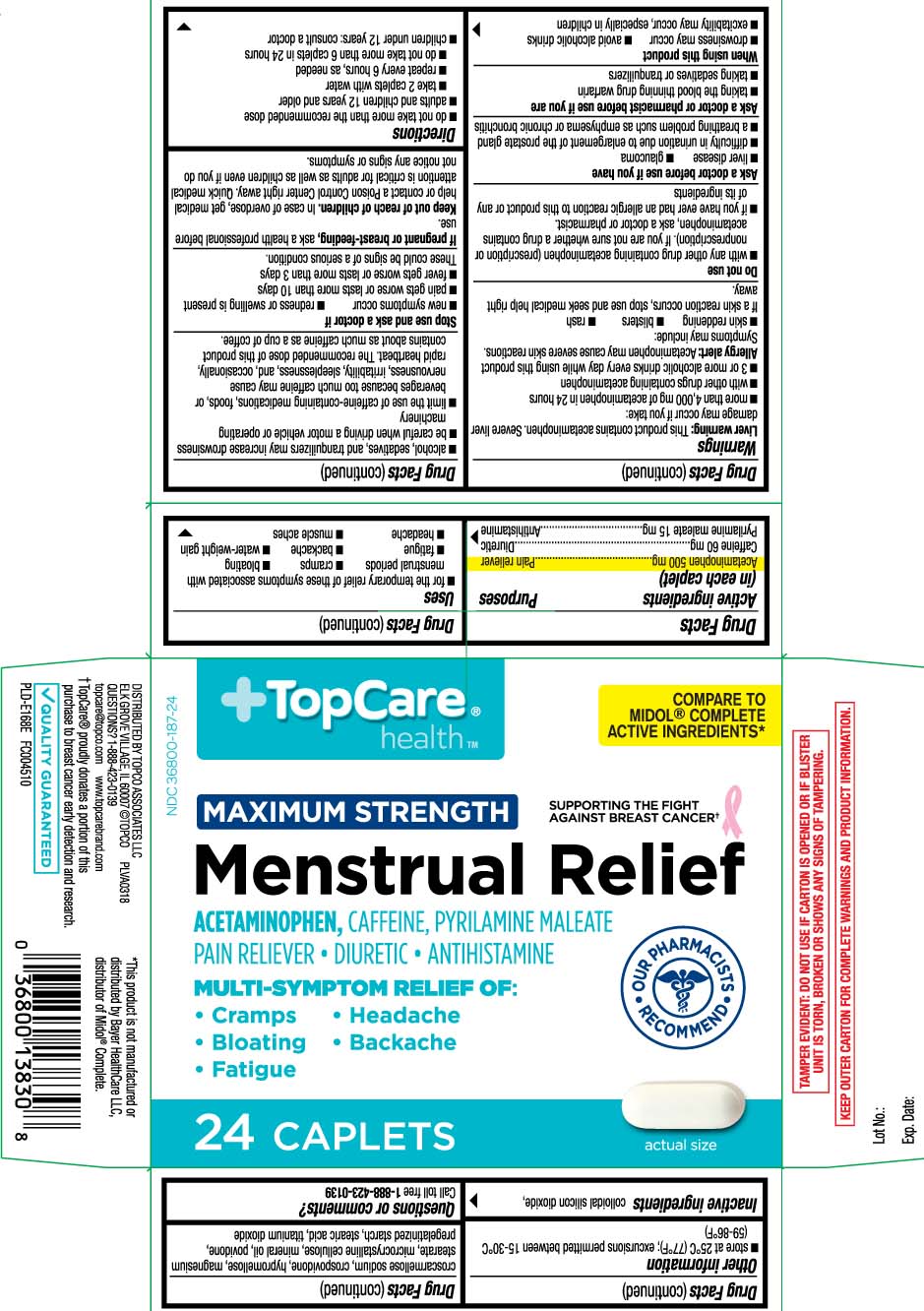
- Active ingredients (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- liver disease
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- excitability may occur, especially in children
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- limit the use of caffeine-containing medications, foods, or beverages because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat. The recommended dose of this product contains about as much caffeine as a cup of coffee.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
COMPARE TO MIDOL® COMPLETE ACTIVE INGREDIENTS*
MAXIMUM STRENGTH SUPPORTING THE FIGHT AGAINST BREAST CANCER†
Menstrual Relief
ACETAMINOPHEN, CAFFEINE, PYRILAMINE MALEATE
PAIN RELIEVER • DIURETIC • ANTIHISTAMINE
MULTI-SYMPTOM RELIEF OF:
- Cramps
- Bloating
- Fatigue
- Backache
- Headache
CAPLETS
*This product is not manufactured or distributed by Bayer HealthCare LLC, distributor of Midol® Complete.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
DISTRIBUTED BY: TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007 ©TOPCO
QUESTIONS? 1-888-423-0139
- Product Label
-
INGREDIENTS AND APPEARANCE
MENSTRUAL RELIEF
acetaminophen, caffeine and pyrilamine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-187 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 60 mg PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 15 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (UNII: 2S7830E561) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape CAPSULE Size 17mm Flavor Imprint Code TCL347 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-187-24 24 in 1 CARTON 03/31/2014 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 03/31/2014 Labeler - TOP CARE (Topco Associates LLC) (006935977)