GRANDPAS THYLOX ACNE TREATMENT WITH SULFUR- sulfur soap
Bradford Soap Works, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Grandpa Soap Co Thylox Acne Treatment-1006
Uses
Acts four ways to improve blemished skin:
- Softens the hard shell of acne blemishes (keratolytic action).
- Helps dissolve and remove blackheads.
- Washes away excess oils which may cause blackheads.
- Freshens skin by its thorough cleansing action.
Keep out of reach of children
Keep out of reach of children. if swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Use twice daily. Work up a lather with hands or washcloth and apply to affected areas without scrubbing. Let dry about one minute, rinse thoroughly, and pat dry.
Inactive Ingredients
Sodium Palmate, Sodium Cocoate*, Water, Glycerin, Fragrance, Titanium Dioxide, Tetrasodium Etidronate, Pentasodium Pentetate, Cosmetic Orange oxide. * May also contain Sodium Palm Kernelate.
|
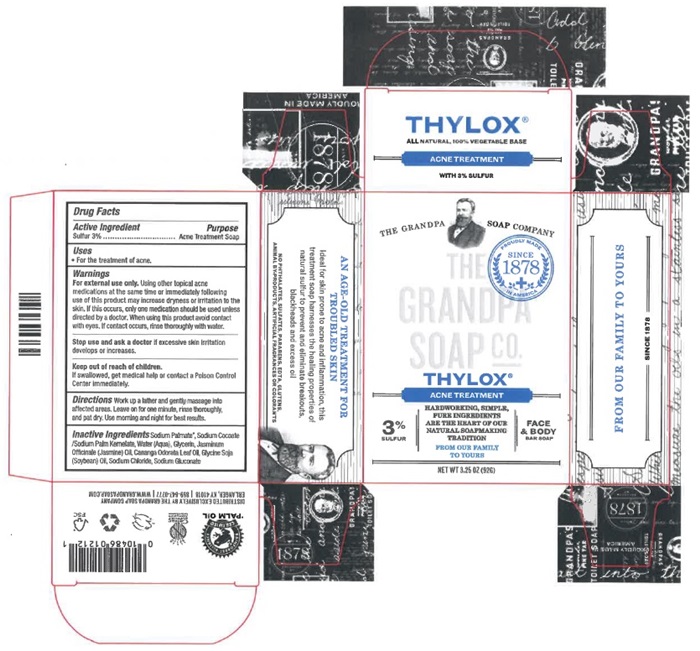
THYLOX® ALL NATURAL, 100% VEGETABLE BASE ACNE TREATMENT WITH 3% SULFUR | |||||||
|
Drug Facts Active Ingredient Purpose Sulfur 3%. .. Acne Treatment Soap Uses
Warnings For external use only. Using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation to the skin. If this occurs, only on medication should be used unless directed by a doctor. When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water. Stop use and ask a doctor if excessive skin irritation develops or increases. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately. Directions Work up a lather and gently massage into affected areas. Leave on for one minute, rinse thoroughly, and pat dry. Use morning and night for best results. Inactive ingredients Sodium Palmate®, Sodium Cocoate/Sodium Palm Kernelate, Water (Aqua), Glycerin, Jasminum Officinale (Jasmine) Oil, Cananga Odorata Leaf Oil, Glyine Soja (Soybean) Oil, Sodium Chloride, Sodium Gluconate |
AN AGE-OLD TREATMENT FOR TROUBLED SKIN Ideal for skin prone to acne and inflammation, this treatment soap harnesses the healing properties of natural sulfur to prevent and eliminate breakouts, blackheads and excess oil. Also phthalates, sulfates, parabene, edta, gluten, animal by-products, artificial fragrances or colorants |
THE GRANDPA SOAP COMPANY THE GRANDPA SOAP CO. THYLOX® ACNE TREATMENT |
FROM OUR FAMILY TO YOURS SINCE 1878 |
||||
|
3% SULFUR |
HARDWORKING, SIMPLE, PURE INGREDIENTS ARE THE HEART OF OUR NATURALSOAPMAKING TRADITION FROM OUR FAMILY TO YOURS NET WT 3.25 OZ (926) |
FACE & BODY BAR SOAP |
|||||
| GRANDPAS THYLOX ACNE TREATMENT WITH SULFUR
sulfur soap |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Bradford Soap Works, Inc. (001201045) |
| Registrant - Bradford Soap Works, Inc. (001201045) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bradford Soap Works, Inc. | 001201045 | manufacture(11118-1006) | |