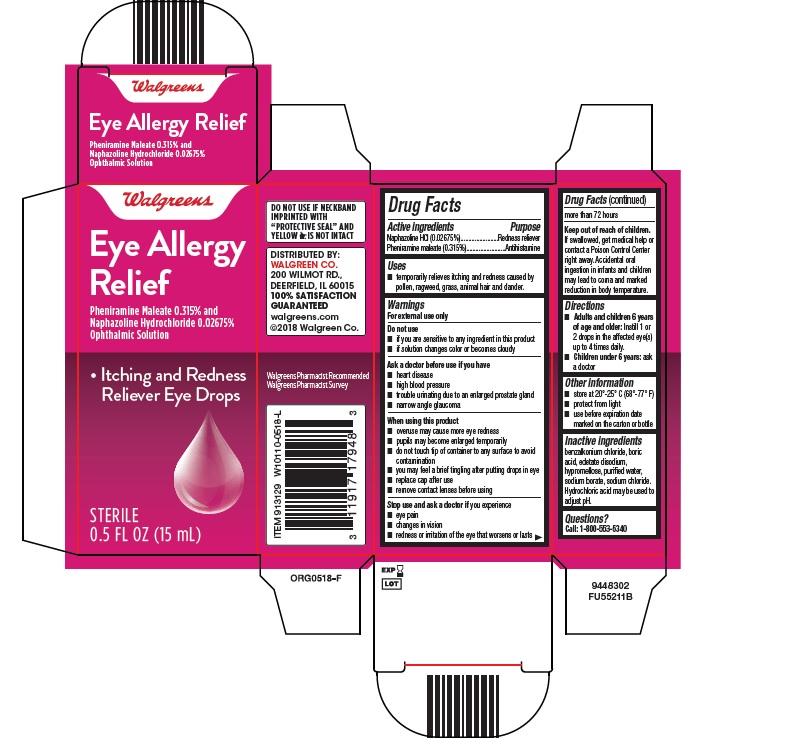
EYE ALLERGY RELIEF- pheniramine maleate and naphazoline hydrochloride solution/ drops
Walgreen Company
----------
Drug Facts
Uses
- •
- temporarily relieves itching and redness caused by pollen, ragweed, grass, animal hair and dander.
Warnings
For external use only
Do not use
- •
- if you are sensitive to any ingredient in this product
- •
- if solution changes color or becomes cloudy
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
- •
- narrow angle glaucoma
When using this product
- •
- overuse may cause more eye redness
- •
- pupils may become enlarged temporarily
- •
- do not touch tip of container to any surface to avoid contamination
- •
- you may feel a brief tingling after putting drops in eye
- •
- replace cap after use
- •
- remove contact lenses before using
Stop use and ask a doctor if you experience
- •
- eye pain
- •
- changes in vision
- •
- redness or irritation of the eye that worsens or lasts more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. Accidental oral ingestion in infants and children may lead to coma and marked reduction in body temperature.
Directions
- •
- Adults and children 6 years
- of age and older: Instill 1 or 2 drops in the affected eye(s) up to 4 times daily.
- •
- Children under 6 years: ask a doctor
Other information
- •
- store at 20°-25° C (68°-77° F)
- •
- protect from light
- •
- use before expiration date marked on the carton or bottle
| EYE ALLERGY RELIEF
pheniramine maleate and naphazoline hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
| Registrant - Bausch & Lomb Incorporated (196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 114406598 | MANUFACTURE(0363-9652) | |
Revised: 10/2021
Document Id: 4c9e1adb-5237-4420-9520-64db471140f1
Set id: bd123ab2-edcf-4d1b-8432-c7364a1f9b6d
Version: 3
Effective Time: 20211008
Walgreen Company