Label: EYE WASH- purified water liquid
- NDC Code(s): 68599-1981-1, 68599-1981-4
- Packager: McKesson
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 18, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
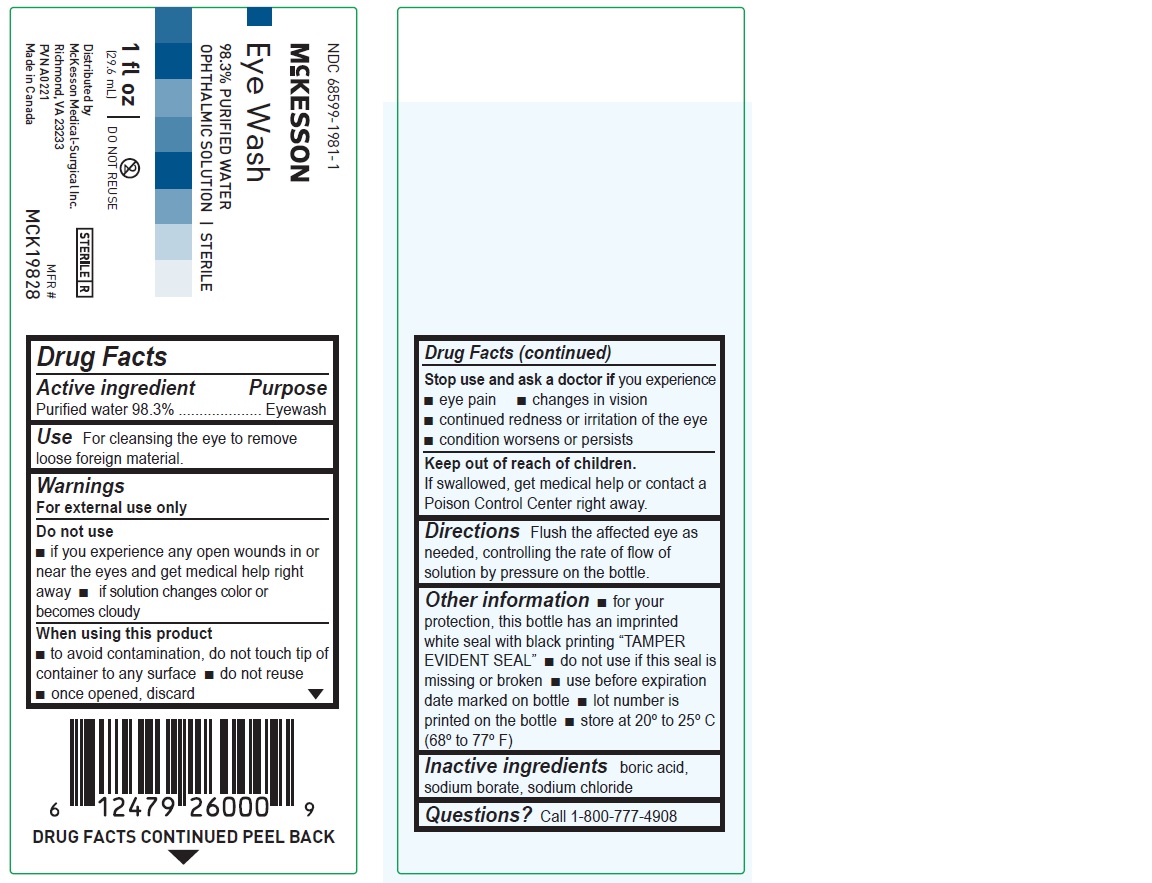
1 fl oz
NDC 68599-1981-1
McKesson
Eye Wash
98.3% PURIFIED WATER
OPHTHALMIC SOLUTION | STERILEMFR# MCK19828
1 fl oz
(29.6 mL)Distributed by
McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN A0221
Made in Canada
1 fl oz eye wash solution
Drug Facts
Active ingredient Purpose
Purified water 98.3% Eyewash
Use
For cleansing the eye to remove loose foreign material
Warnings
For external use only
Do not use
• if you experience any open wounds in or near the eyes and get medical help right away
• if solution changes color or becomes cloudy
When using this product
• to avoid contamination, do not touch tip of container to any surface
• do not reuse
• once opened, discard
Stop use and ask a doctor if you experience
• eye pain
• changes in vision
• continued redness or irritation of the eye
• condition worsens or persists
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Other information
• for your protection, this bottle has an imprinted white seal with black printing “TAMPER EVIDENT SEAL”
• do not use if this seal is missing or broken
• use before expiration date marked on bottle
• lot number is printed on the bottle
• store at 20º to 25ºC (68º to 77ºF)
Inactive ingredients
boric acid, sodium borate, sodium chloride
Questions? Call 1-800-777-4908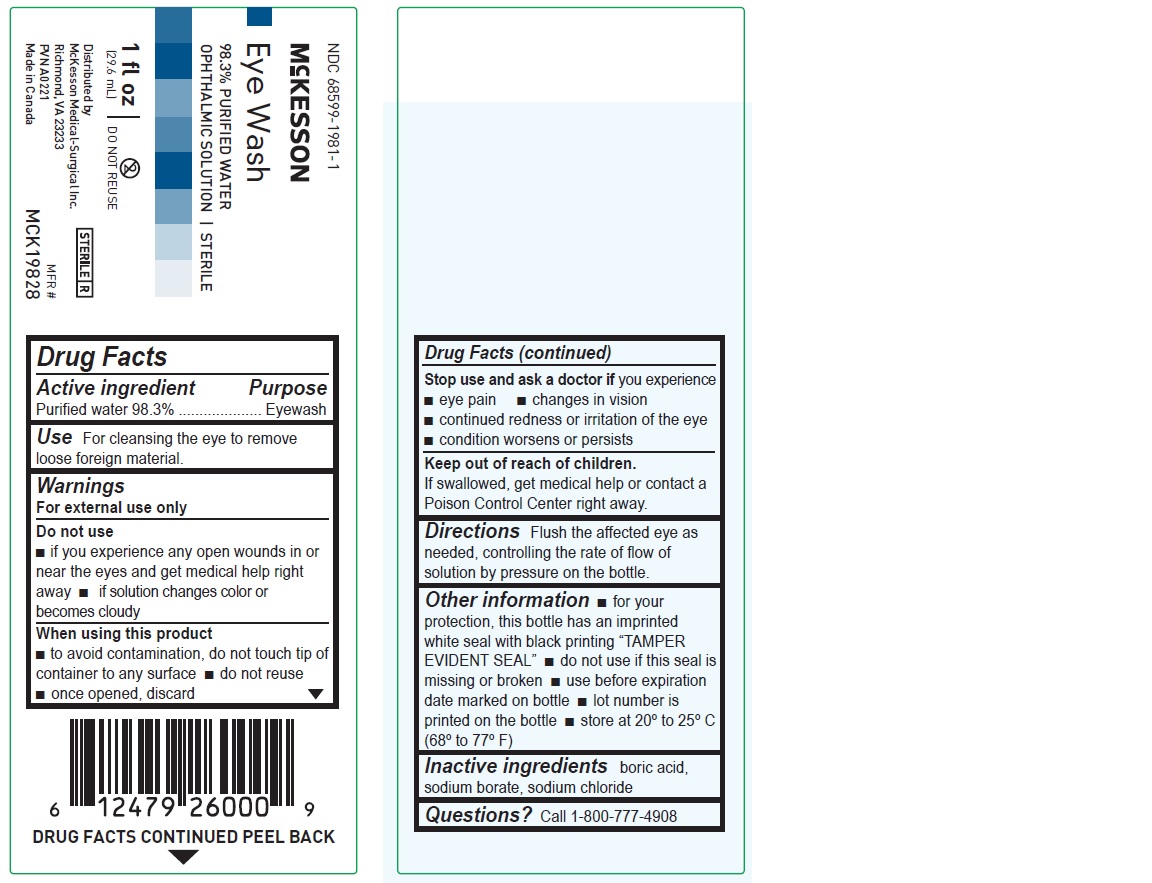

-
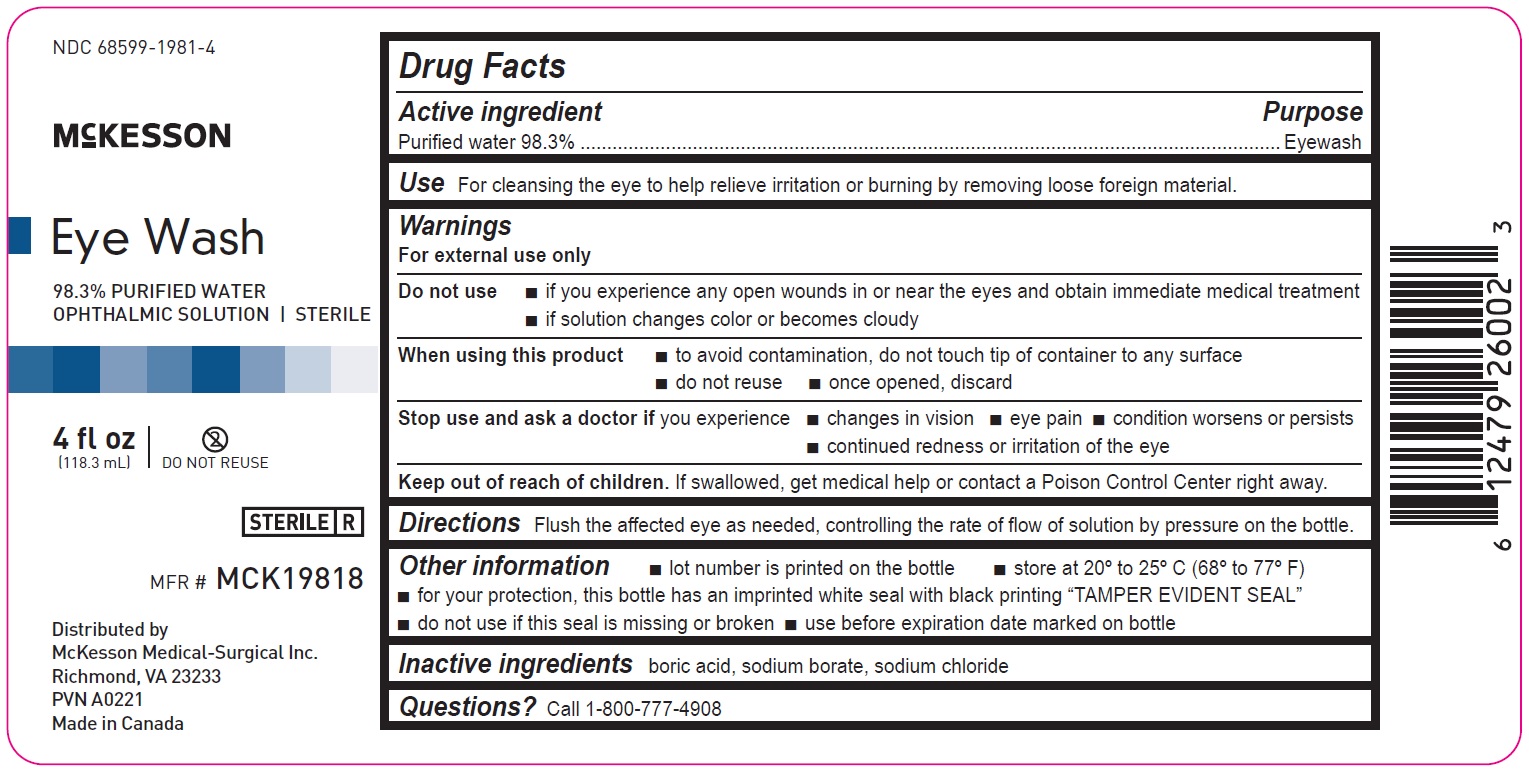
4 fl oz
68599-1981-4
McKesson
Eye Wash
98.3% PURIFIED WATER
OPHTHALMIC SOLUTION | STERILEMFR# MCK19818
4fl oz
(118.3mL)
Distributed by
McKesson Medical-Surgical Inc.
Richmond, VA 23233
PVN A0221
Made in Canada
4 fl oz eye wash solution
Drug Facts
Drug Facts
Active ingredient Purpose
Purified water 98.3% Eyewash
Use
For cleansing the eye to help relieve irritation or burning by removing loose foreign material
Warnings
For external use only
Do not use
• if you experience any open wounds in or near the eyes and obtain immediate medical treatment
• if solution changes color or becomes cloudy
When using this product
• to avoid contamination, do not touch tip of container to any surface
• do not reuse
• once opened, discard
Stop use and ask a doctor if you experience
• changes in vision
• eye pain
• condition worsens or persists
• continued redness or irritation of the eye
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Other information
• lot number is printed on the bottle
• store at 20º to 25ºC (68º to 77ºF)
• for your protection, this bottle has an imprinted white seal with black printing “TAMPER EVIDENT SEAL”
• do not use if this seal is missing or broken
• use before expiration date marked on bottle
Inactive ingredients
boric acid, sodium borate, sodium chloride
Questions? Call 1-800-777-4908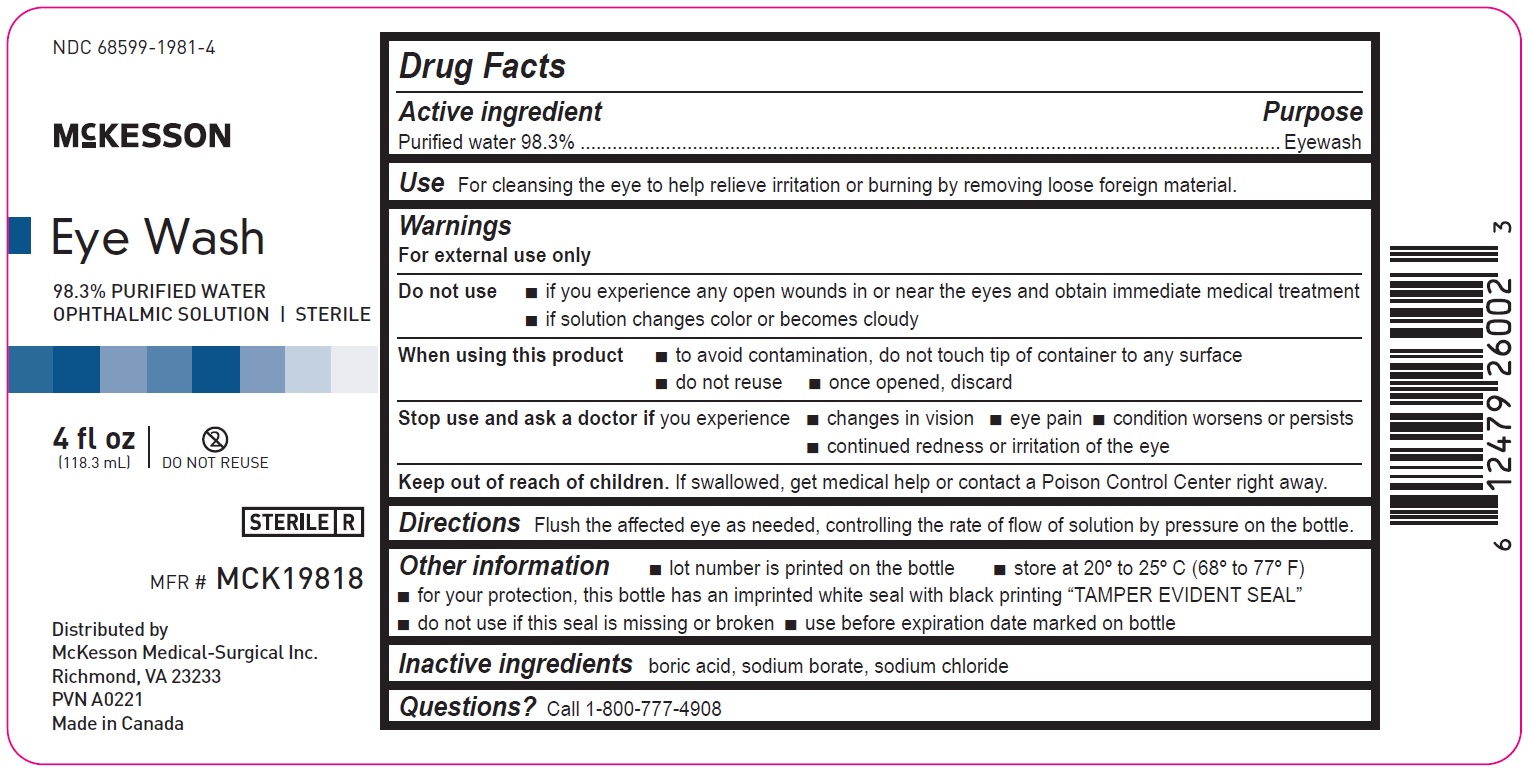

-
INGREDIENTS AND APPEARANCE
EYE WASH
purified water liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-1981 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.3 mL in 100 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-1981-1 29.6 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 04/01/2021 2 NDC:68599-1981-4 118.3 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 04/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 04/01/2021 Labeler - McKesson (023904428) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals 205477792 manufacture(68599-1981)