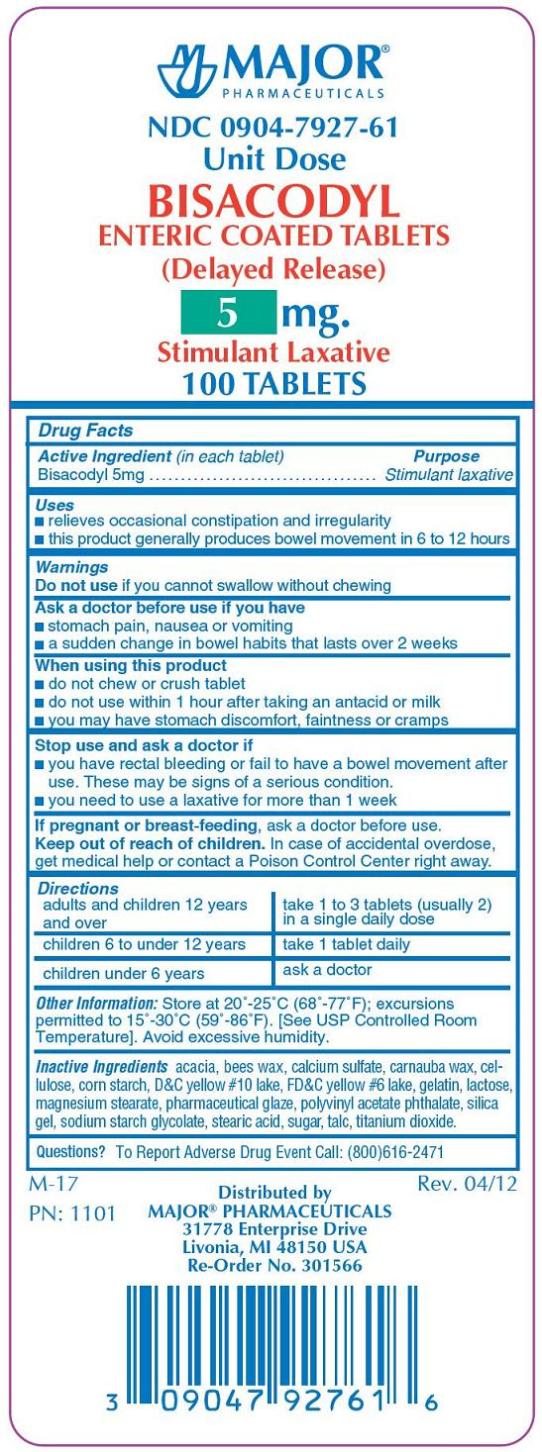
BISACODYL ENTERIC COATED- bisacodyl tablet, coated
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Bisacodyl Enteric Coated Tablet
Use(s)
-relieves occasional constipation and irregularity
-this product generally produces bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if
-you have a sudden change in bowel habits that lasts more than 2 weeks
-stomach pain, nausea or vomiting
When using this product
-do not use within 1 hour after taking an antacid or milk
-do not chew or crush tablet(s)
-you may have stomach discomfort, faintness or cramps
Directions
-take with a glass of water
adults and children 12 years and over- take 1 to 3 tablets in a single daily dose
children 6 to under 12 years of age - take 1 tablet in a single daily dose
children under 6 years of age - ask a doctor
Other information
-store at 25 degrees C (77 degrees F) excursions permitted between 15 degrees-30 degrees C (59 degrees-86 degrees F)
-use by expiration date on package
-protect from excessive humidity
Inactive ingredients
acacia, bees wax, calcium sulfate, carnauba wax, cellulose, corn starch, D&C Yellow No. 10 lake, FD&C Yellow No. 6 lake, gelatin, lactose, magnesium stearate, pharmaceutical glaze, polyvinyl acetate phthalate, silica gel, sodium starch glycolate, stearinc acid, sugar, talc, titanium dioxide.
| BISACODYL
ENTERIC COATED
bisacodyl tablet, coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |