TRAUMEEL RX- traumeel rx tablet
MediNatura Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Traumeel Rx Tablet
INDICATIONS & USAGE SECTION
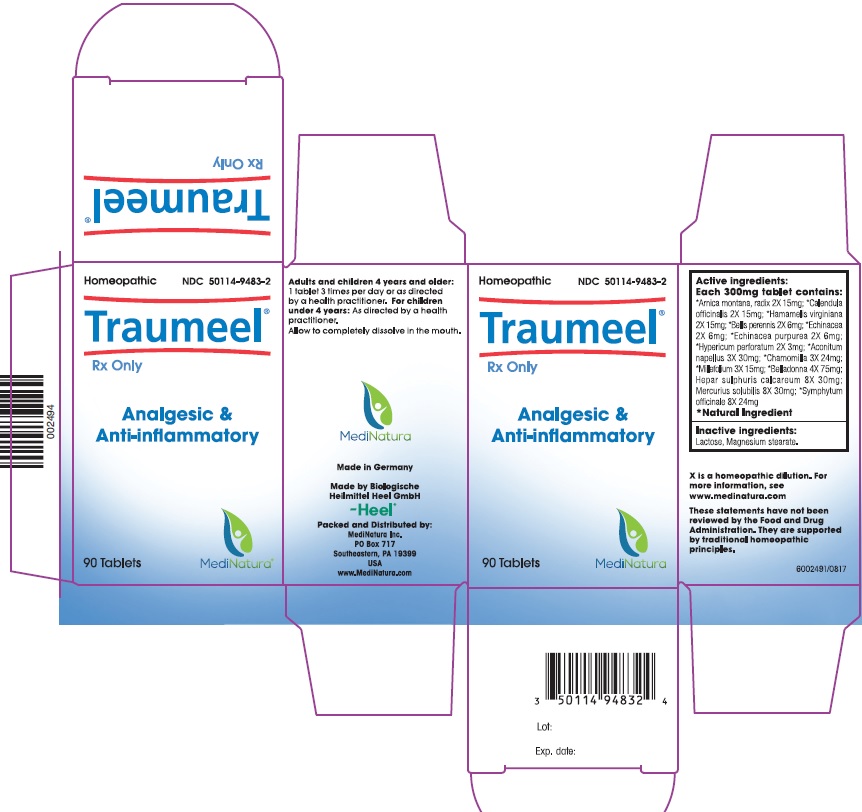
Traumeel ® tablets are an analgesic / anti-inflammatory homeopathic drug product indicated for the treatment of
injuries, inflammatory and degenerative conditions of the musculoskeletal system and for the relief of associated
symptoms such as pain.
Dosage
Adults and children 4 years and older: 1 tablets per day 3 times per day or as directed by a health practitioner.
For children under 4 years: As directed by a health practitioner.
Allow to completely dissolve in the mouth.
Ingredients
Active Ingredients:
Ingredient name Potency Quantity Final dilution
Aconitum napellus 3X 30 mg 4X
Arnica montana, radix 2X 15 mg 3.3X
Belladonna 4X 75 mg 4.6X
Bellis perennis 2X 6 mg 3.7X
Calendula officinalis 2X 15 mg 3.3X
Chamomilla 3X 24 mg 4.1X
Echinacea 2X 6 mg 3.7X
Echinacea purpurea 2X 6 mg 3.7X
Hamamelis virginiana 2X 15 mg 3.3X
Hepar sulphuris calcareum 8X 30 mg 9X
Hypericum perforatum 2X 3 mg 4X
Mercurius solubilis 8X 30 mg 9X
Millefolium 3X 15 mg 4.3X
Symphytum officinale 8X 24 mg 9.1X
| TRAUMEEL RX
traumeel rx tablet |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MediNatura Inc (102783016) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biologische Heilmittel Heel | 315635359 | manufacture(50114-9483) | |