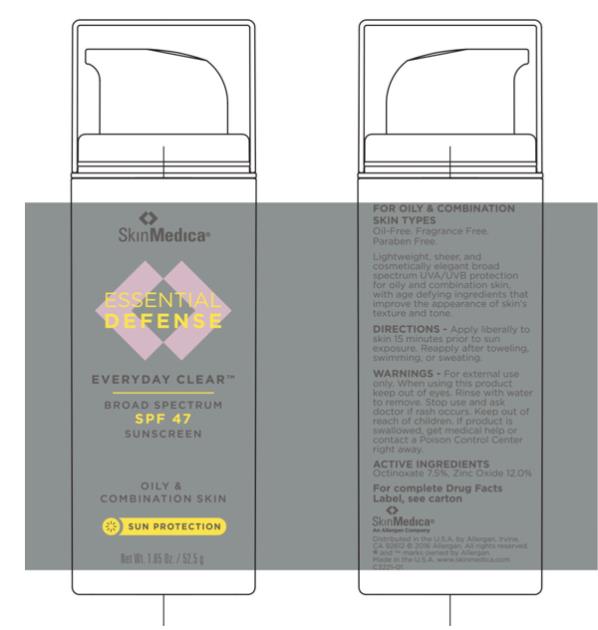
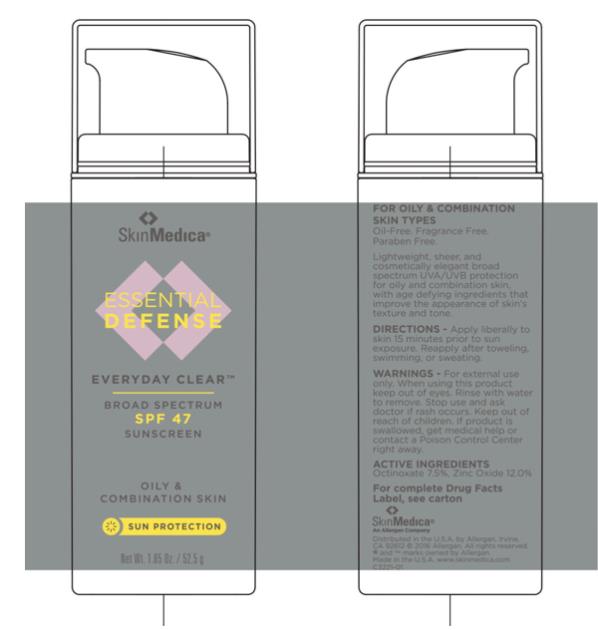
Label: ESSENTIAL DEFENSE EVERYDAY CLEAR BROAD SPECTRUM SPF 47 SUNSCREEN- zinc oxide and octinoxate lotion
- NDC Code(s): 0023-5738-18, 0023-5738-25
- Packager: Allergan, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 2, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Helps prevent sunburn
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
-
Sun Protection Measures Spending time in the sun increases the risk of skin cancer, premature skin aging and other skin damage. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including limiting time in the sun from 10 a.m.-2 p.m., and wearing protective clothing
- Children under 6 months: Ask a doctor
- Apply liberally 15 minutes before sun exposure
-
Inactive ingredients
Water, Cyclopentasiloxane, Niacinamide, Oleth-3 Phosphate, Octyldodecyl Neopentanoate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Glycerin, Camellia Sinensis Leaf Extract, Polygonum Aviculare Extract, Tocopheryl Acetate, Sodium Hyaluronate, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Triethoxycaprylylsilane, Polyisobutene, PEG-7 Trimethylolpropane Coconut Ether, Butylene Glycol, Disodium EDTA, Sodium Hydroxide, Citric Acid, Caprylyl Glycol, Sorbic Acid, Phenoxyethanol, Ethylhexylglycerin
- Other information
- Questions or comments?
- Principal Display Panel - Carton Label
- Principal Display Panel - Tube Label
-
INGREDIENTS AND APPEARANCE
ESSENTIAL DEFENSE EVERYDAY CLEAR BROAD SPECTRUM SPF 47 SUNSCREEN
zinc oxide and octinoxate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0023-5738 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) NIACINAMIDE (UNII: 25X51I8RD4) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) POLYISOBUTYLENE (1300 MW) (UNII: 241BN7J12Y) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GREEN TEA LEAF (UNII: W2ZU1RY8B0) EDETATE DISODIUM (UNII: 7FLD91C86K) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POLYGONUM AVICULARE TOP (UNII: ZCD6009IUF) SODIUM HYDROXIDE (UNII: 55X04QC32I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SORBIC ACID (UNII: X045WJ989B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0023-5738-18 1 in 1 CARTON 03/16/2016 1 52.5 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0023-5738-25 6 in 1 CARTON 03/16/2016 2 7.1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/16/2016 Labeler - Allergan, Inc. (144796497)