HARMONY HEMP NEUROCOMFORT PAIN RELIEF LOTION500- lidocaine hcl usp, 4% lotion
HARMONY HEMP NEUROCOMFORT PAIN RELIEF GEL 1000- lidocaine hcl usp, 4% gel
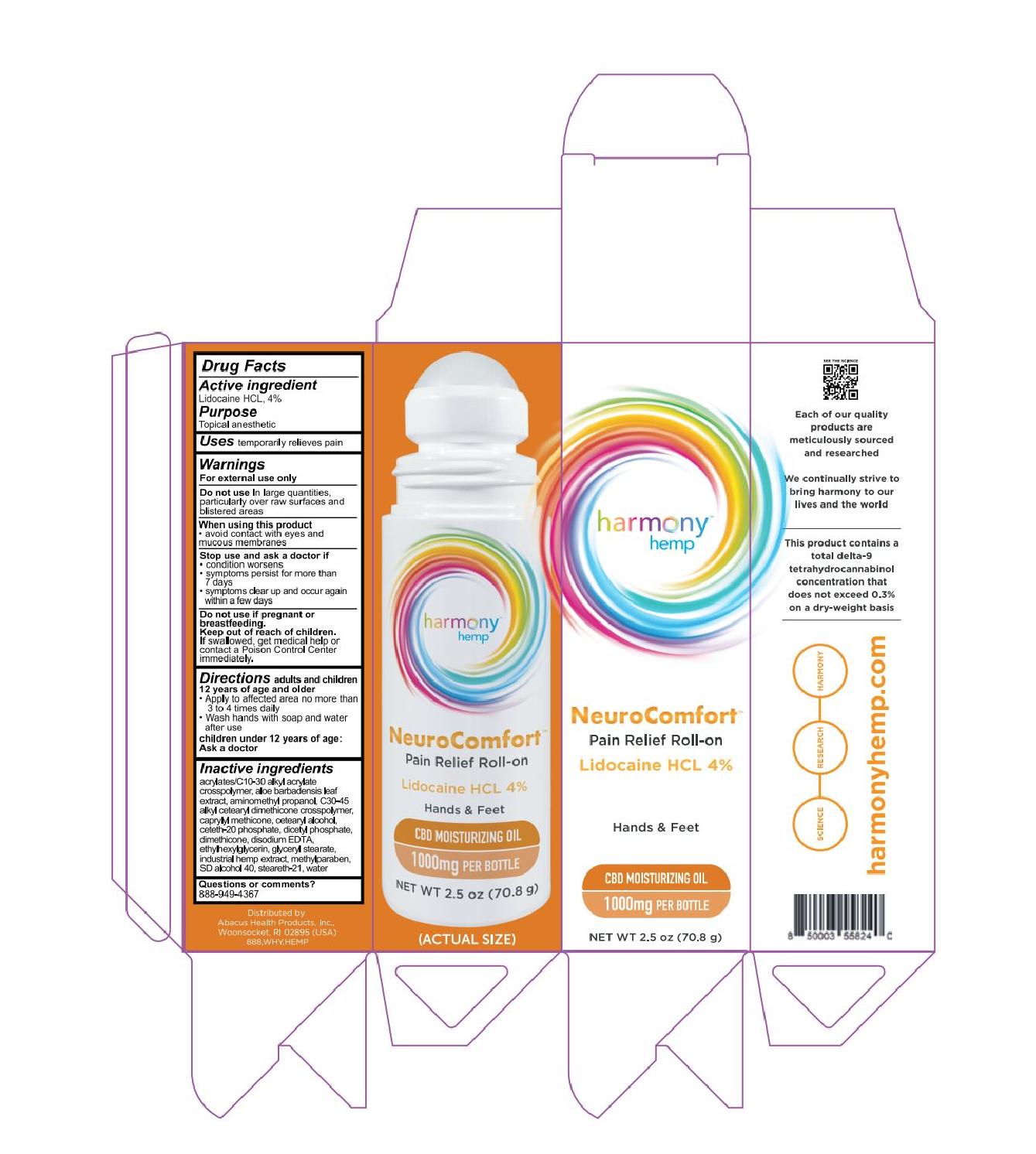
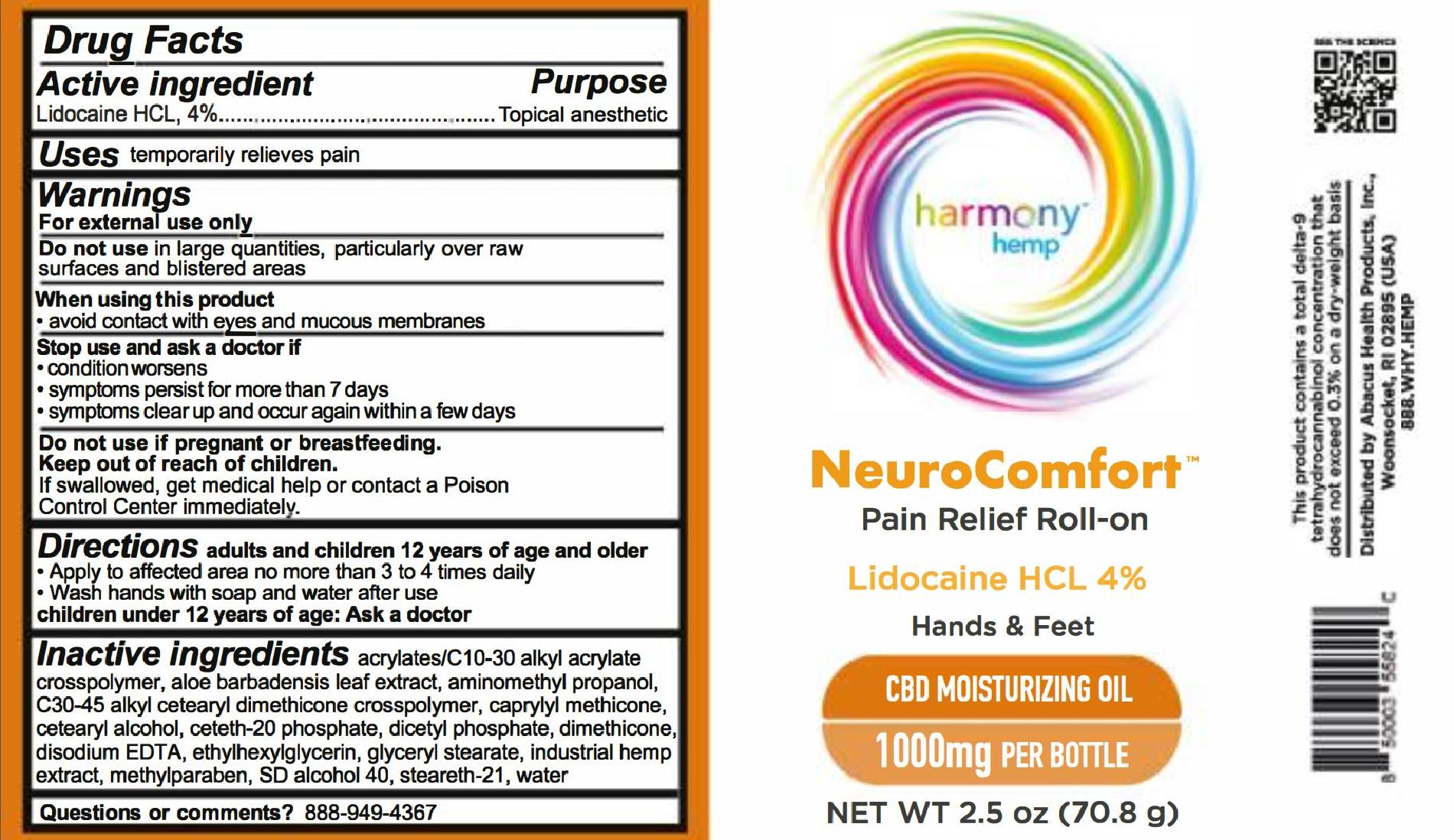
HARMONY HEMP NEUROCOMFORT PAIN RELIEF ROLL-ON 1000- lidocaine hcl usp, 4% gel
HARMONY HEMP NEUROCOMFORT PAIN RELIEF ROLL-ON 500- lidocaine hcl usp, 4% gel
HARMONY HEMP NEUROCOMFORT PAIN RELIEF GEL 500- lidocaine hcl usp, 4% gel
Abacus Health Products, Inc.
----------
NeuroComfort Line
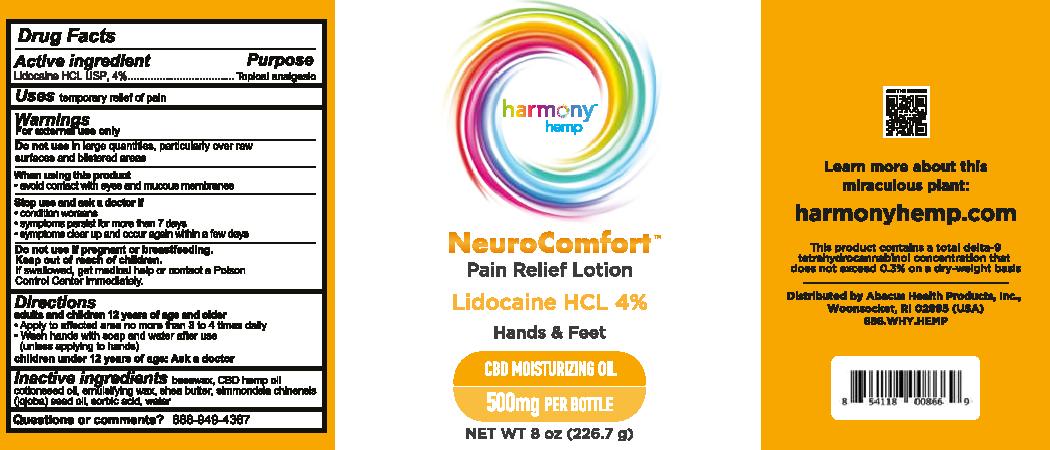
For NDC 73096-065 Harmony Hemp NeuroComfort Pain Relief Lotion 500
Harmony Hemp
NeuroComfort
Pain Relief Lotion
Lidocaine HCL 4%
Hands & Feet
CBD Moisturizing Oil
500mg per bottle
Net Wt 8 oz (226.7 g)
For external use only
adults and children 12 years of age and older
- Apply to affected area no more than 3 to 4 times daily
- Wash hands with soap and water after use (unless applying to hands)
children under 12 years of age: Ask a doctor
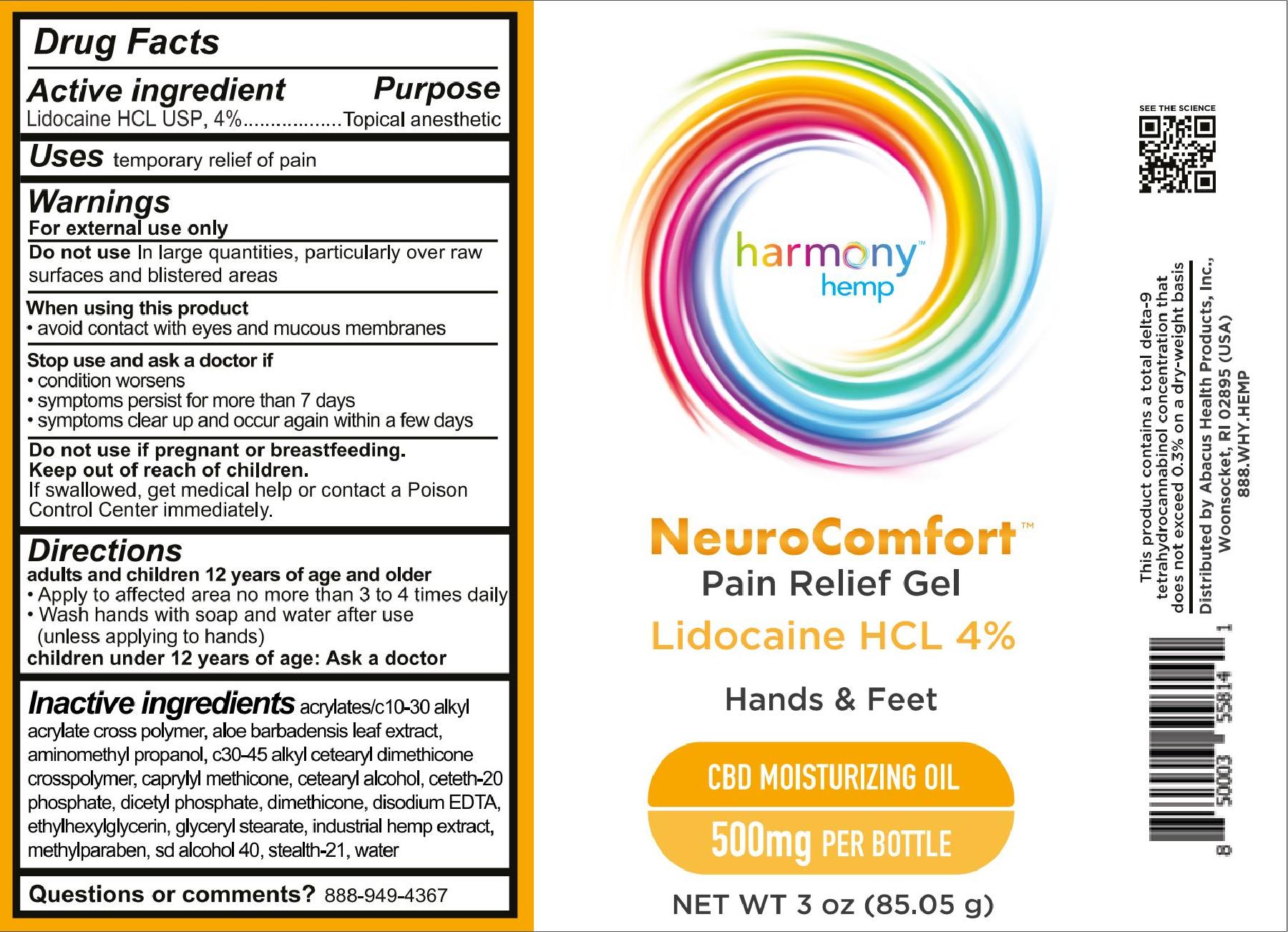
For NDC 73096-066 Harmony Hemp NeuroComfort Pain Relief Gel 500
Harmony Hemp
NeuroComfort
Pain Relief Gel
Lidocaine HCL 4%
Hands & Feet
CBD Moisturizing Oil
500mg per bottle
Net Wt 3 oz (85.05g)
adults and children 12 years of age and older
- Apply to affected area no more than 3 to 4 times daily.
- Wash hands with soap and water after use (unless applying to hands)
children under 12 years of age: Ask a doctor
acrylates/c10-30 alkyl acrylate cross polymer, aloe barbadensis leaf extract, aminomethyl propanol, c30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, industrial hemp extract, methylparaben, sd alcohol 40, stealth-21, water
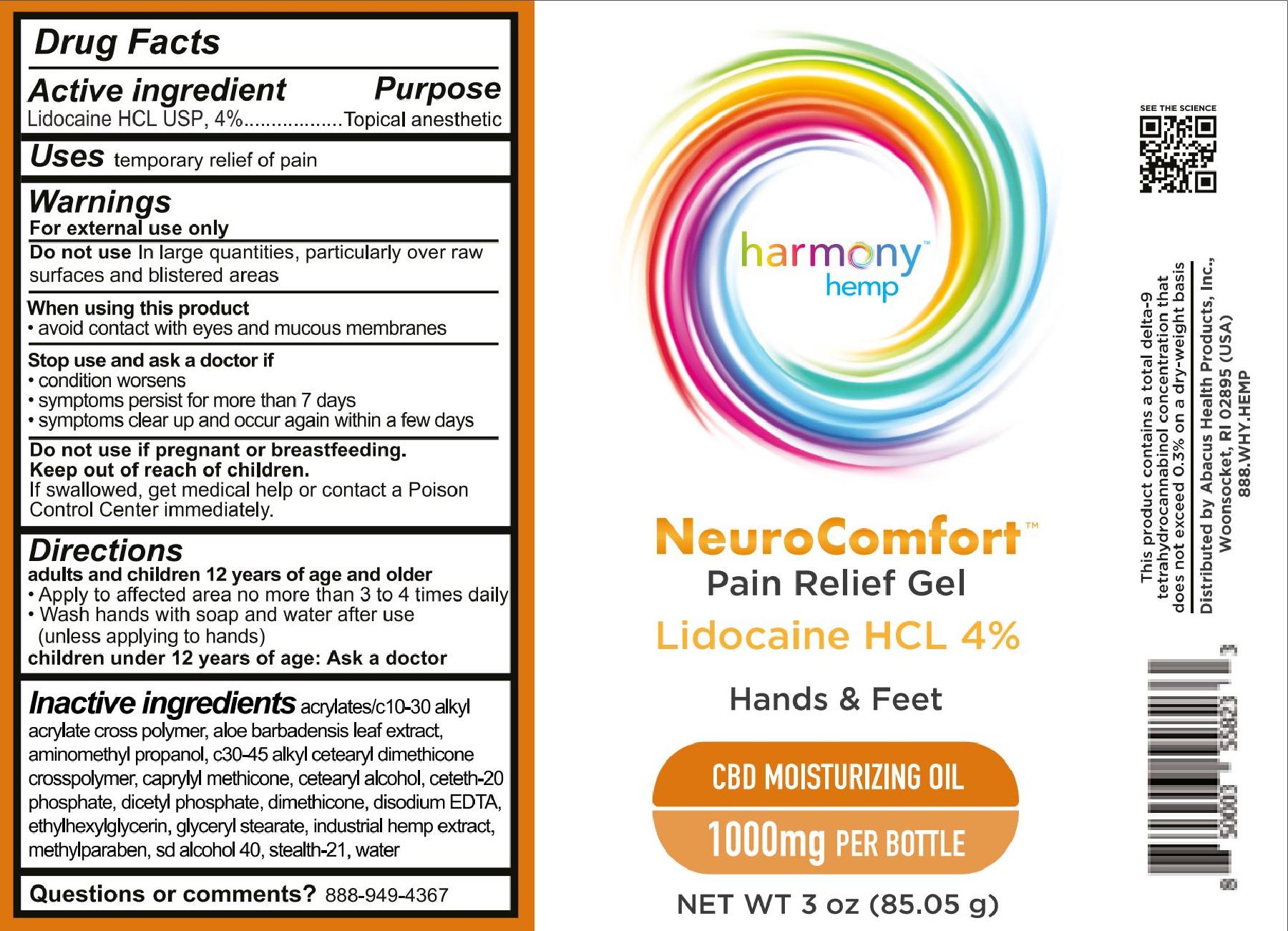
For NDC 73096-067 Harmony Hemp NeuroComfort Pain Relief Gel 1000
Harmony Hemp
NeuroComfort
Pain Relief Gel
Lidocaine HCL 4%
Hands & Feet
CBD Moisturizing Oil
1000mg per bottle
Net wt 3 oz (85.05 g)
For external use only
adults and children 12 years of age and older
- Apply to affected area no more than 3 to 4 times daily.
- Wash hands with soap and water after use (unless applying to hands)
children under 12 years of age: Ask a doctor
acrylates/c10-30 alkyl acrylate cross polymer, aloe barbadensis leaf extract, aminomethyl propanol, c30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, industrial hemp extract, methylparaben, sd alcohol 40, stealth-21, water
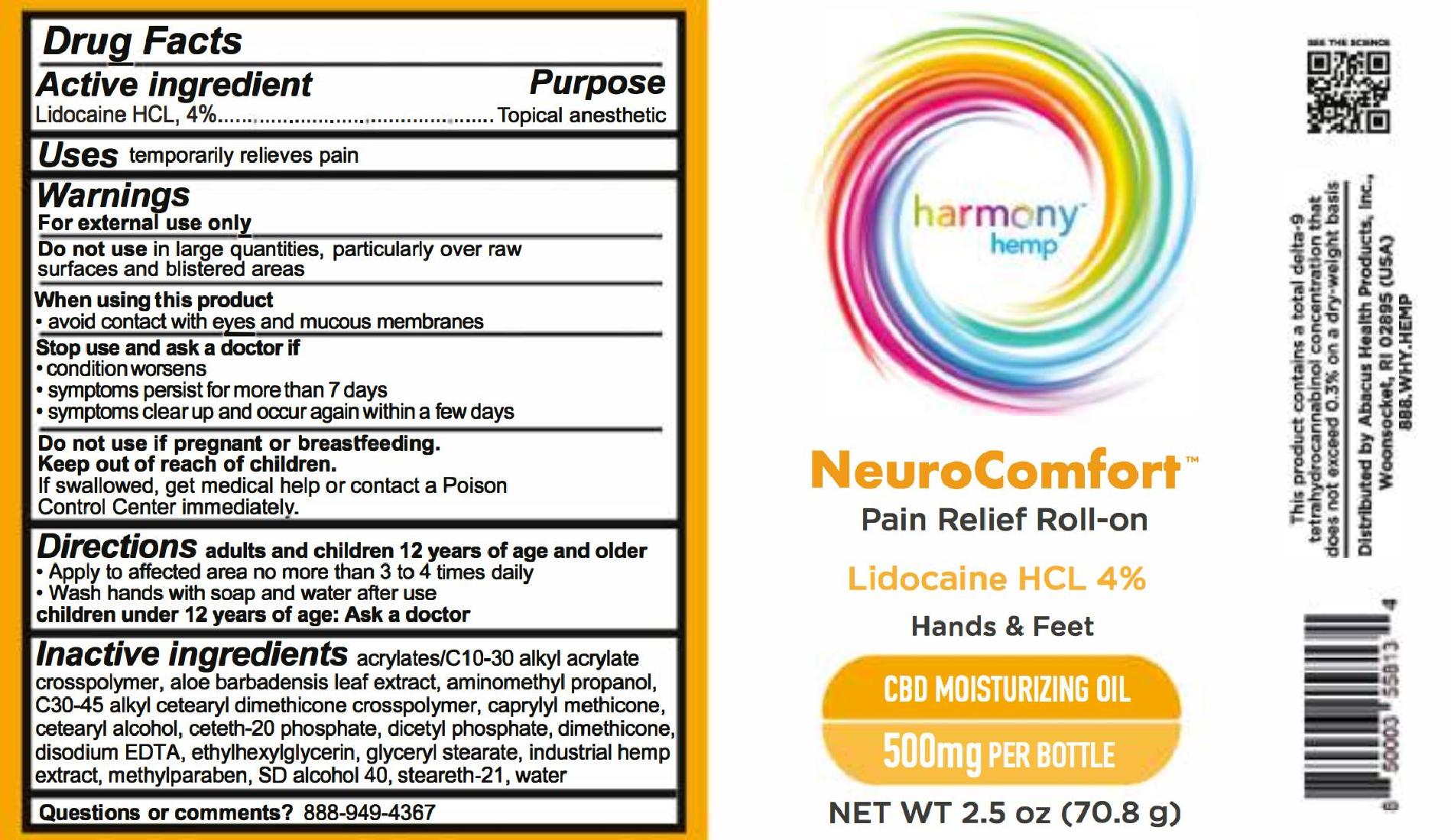
For NDC 73096-068 Harmony Hemp NeuroComfort Pain Relief Roll-on 500
Harmony Hemp
NeuroComfort
Pain Relief Roll-on
LIdocaine HCL 4%
Hands & Feet
CBD Moisturizing Oil
500mg per bottle
Net wt 2.5 oz (70.8 g)
adults and children 12 years of age and older
- Apply to affected area no more than 3 to 4 times daily
- Wash hands with soap and water after use
children under 12 years of age: Ask a doctor
acrylates/c10-30 alkyl acrylate cross polymer, aloe barbadensis leaf extract, aminomethyl propanol, c30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, industrial hemp extract, methylparaben, sd alcohol 40, stealth-21, water
For NDC 73096-069 Harmony Hemp NeuroComfort Pain Relief Roll-on 1000
Harmony Hemp
NeuroComfort
Pain Relief Roll-on
Lidocaine HCL 4%
Hands & Feet
CBD Moisturizing Oil
1000mg per bottle
Net wt 2.5 oz (70.8 g)
adults and children 12 years of age and older
- Apply to affected area no more than 3 to 4 times daily
- Wash hands with soap and water after use
children under 12 years of age: Ask a doctor
acrylates/c10-30 alkyl acrylate cross polymer, aloe barbadensis leaf extract, aminomethyl propanol, c30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, industrial hemp extract, methylparaben, sd alcohol 40, stealth-21, water
| HARMONY HEMP NEUROCOMFORT PAIN RELIEF LOTION500
lidocaine hcl usp, 4% lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HARMONY HEMP NEUROCOMFORT PAIN RELIEF GEL 1000
lidocaine hcl usp, 4% gel |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| HARMONY HEMP NEUROCOMFORT PAIN RELIEF ROLL-ON 1000
lidocaine hcl usp, 4% gel |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| HARMONY HEMP NEUROCOMFORT PAIN RELIEF ROLL-ON 500
lidocaine hcl usp, 4% gel |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| HARMONY HEMP NEUROCOMFORT PAIN RELIEF GEL 500
lidocaine hcl usp, 4% gel |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Abacus Health Products, Inc. (116931574) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aidance Scientific, Inc, DBA Aidance Skincare & Topical Solutions | 018950611 | manufacture(73096-065) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Inspec Solutions | 081030372 | manufacture(73096-066, 73096-067, 73096-068, 73096-069) | |