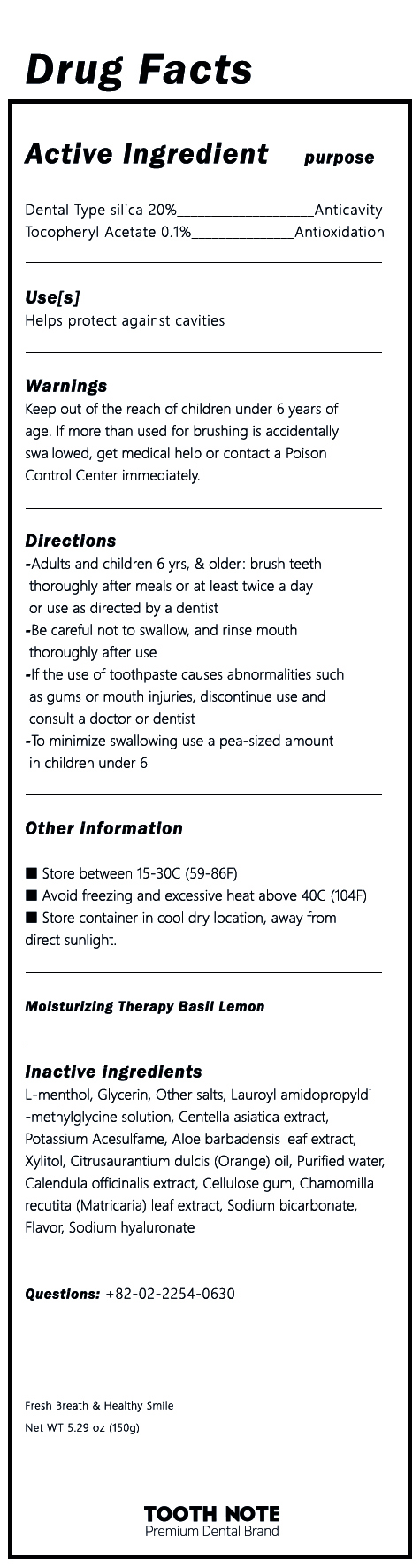
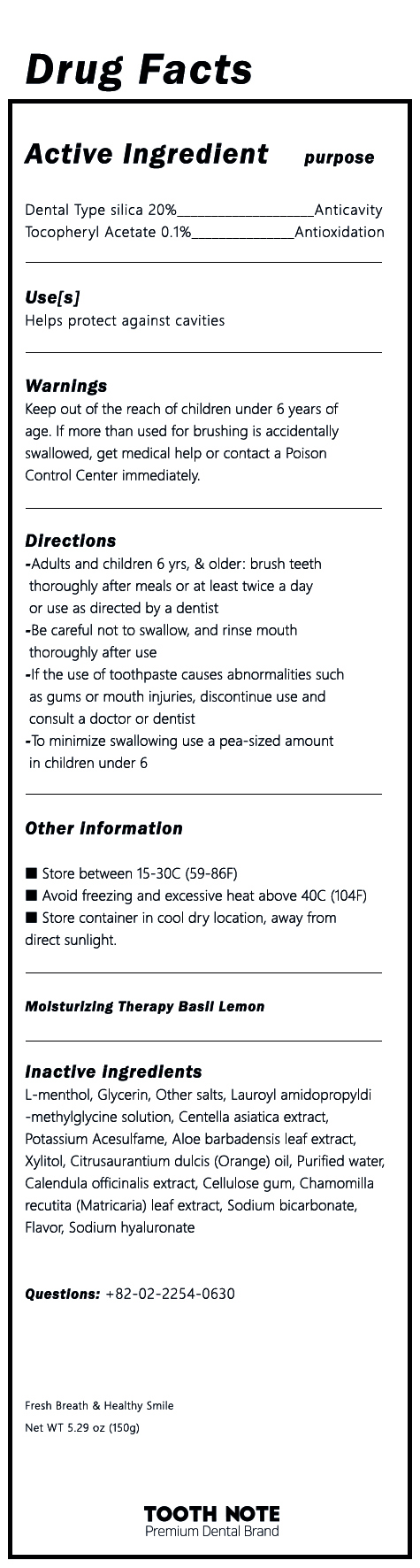
Label: TOOTH NOTE MOISTURIZING THERAPY TOOTH LEMON BASIL- dental type silica, tocopheryl acetate paste, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 81581-0001-1 - Packager: GI BILLIONS Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 23, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
L-menthol, Glycerin, Other Salts, Lauroyl Amidopropyldi-methylglycine solution, Centella asiatica extract, Potassium Acesulfame, Aloe barbadensis leaf extract, Xylitol, Citrusaurantium dulcis (Orange) oil, Purified water, Calendula officinalis extract, Cellulose gum, Chamomilla recutita (Matricaria) leaf extract, Sodium bicarbonate, Flavor, Sodium hyaluronate
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1) Be careful not to swallow, and rinse mouth thoroughly after use
2) If the use of toothpaste causes abnormalities such as gums or mouth injuries, discontinue use and consult a doctor or dentist
3) If you are a child under 6 years of age, use a small amount of toothpaste as small as pea per use, and use under the supervision of a guardian to avoid sucking or swallowing
4 If a child under 6 years of age swallowed large quantities, consult a physician or dentist immediately
5) Keep out of the reach of children under 6 years - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOOTH NOTE MOISTURIZING THERAPY TOOTH LEMON BASIL
dental type silica, tocopheryl acetate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81581-0001 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 20 g in 100 g .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) WATER (UNII: 059QF0KO0R) LEVOMENTHOL (UNII: BZ1R15MTK7) CENTELLA ASIATICA (UNII: 7M867G6T1U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81581-0001-1 150 g in 1 TUBE; Type 0: Not a Combination Product 02/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2021 Labeler - GI BILLIONS Co.,Ltd. (695631555) Registrant - GI BILLIONS Co.,Ltd. (695631555) Establishment Name Address ID/FEI Business Operations Biostech Co., Ltd. 687294330 manufacture(81581-0001) Establishment Name Address ID/FEI Business Operations GI BILLIONS Co.,Ltd. 695631555 label(81581-0001)