NITROGLYCERIN- nitroglycerin capsule
PD-Rx Pharmaceuticals, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
NITROGLYCERIN 2.5 MG, 6.5 MG and 9.0 MG
HOW SUPPLIED:
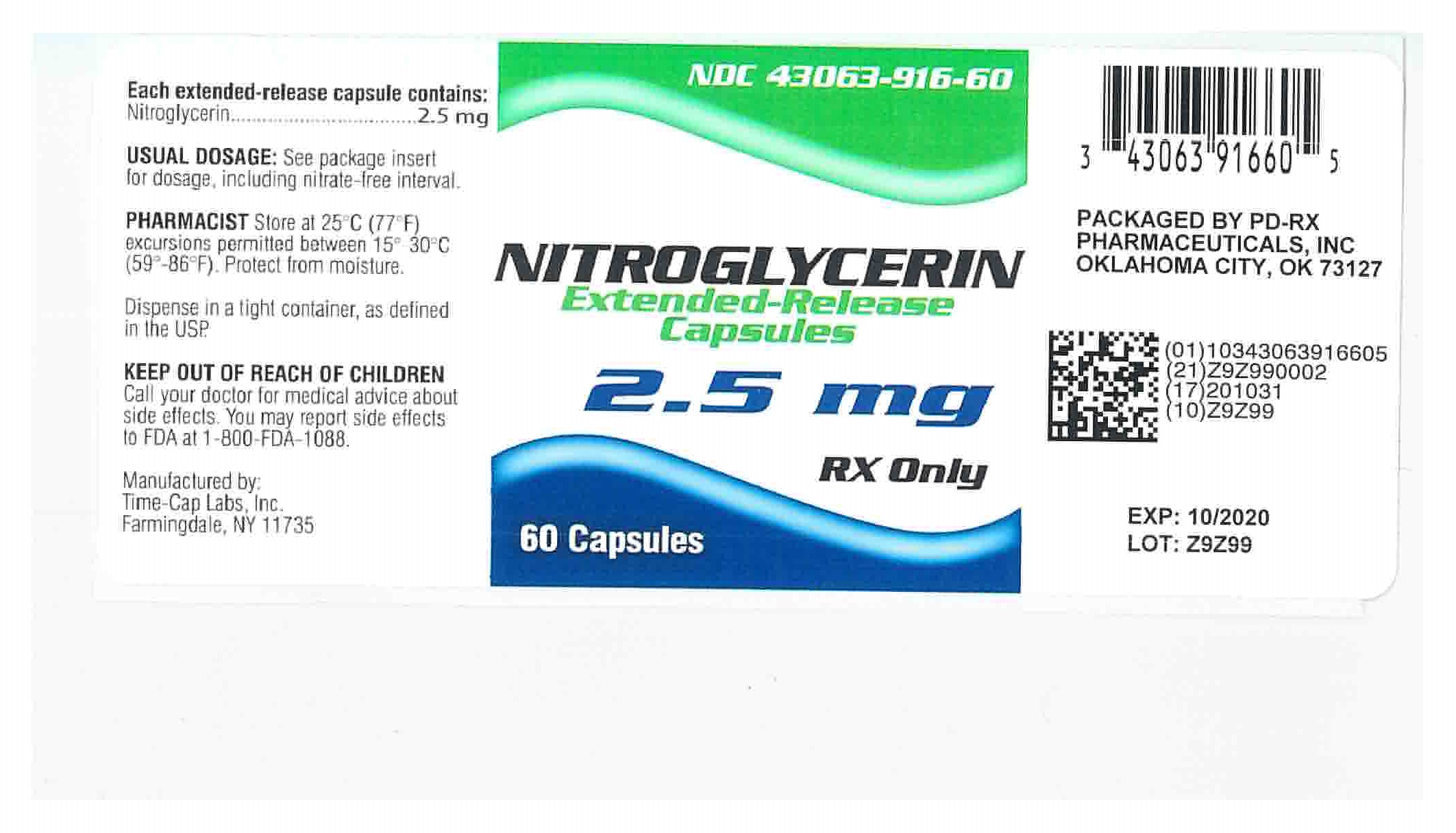
2.5 mg Nitroglycerin Extended-Release (Pink and Clear Capsules imprinted TCL-1221) in bottles of 60's NDC 43063-916-60
HOW SUPPLIED
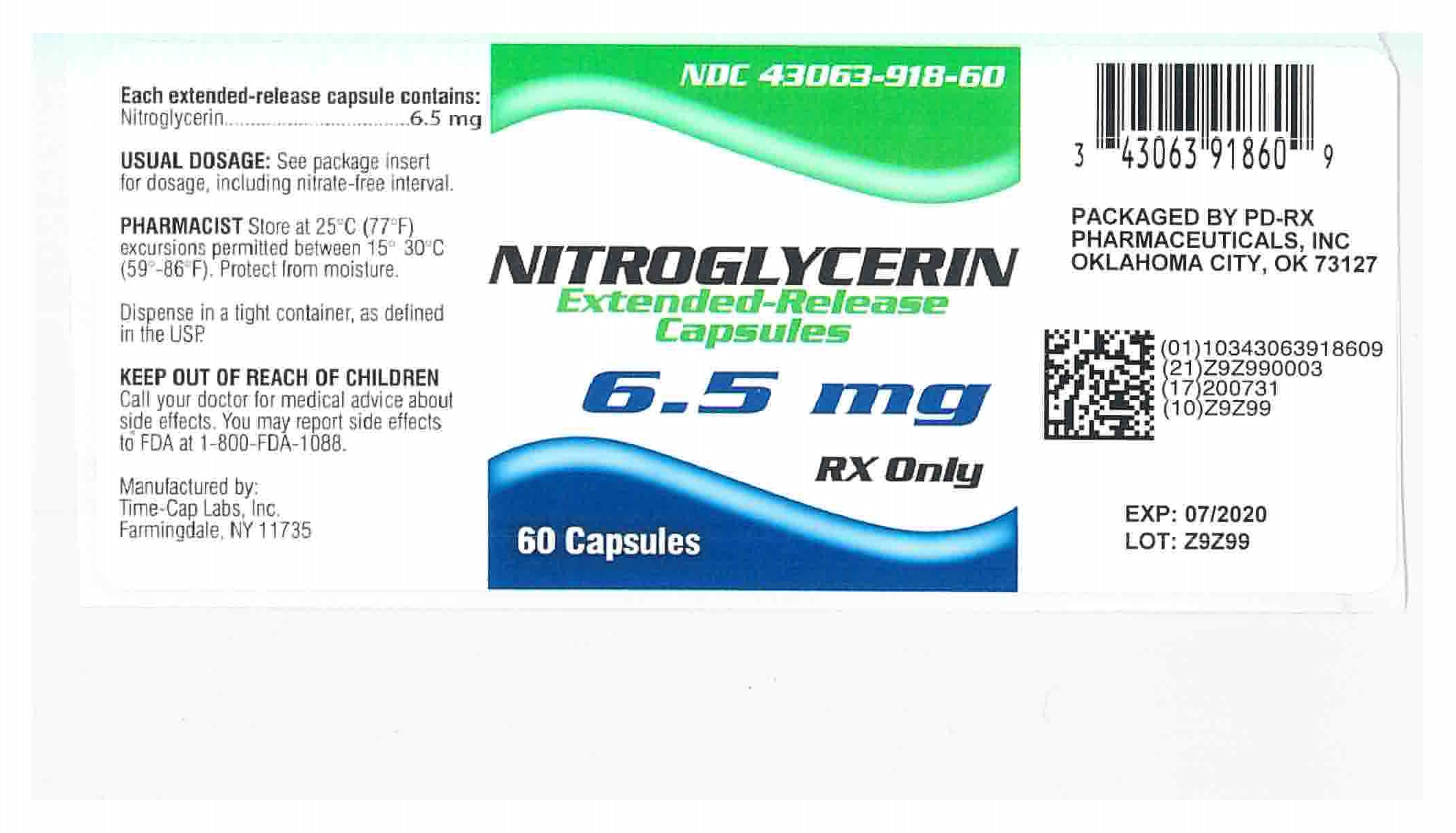
6.5 mg Nitroglycerin Extended-Release Capsules Blue/Yellow imprinted TCL1222 in bottles of 60's NDC 43063-918-60
| NITROGLYCERIN
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| NITROGLYCERIN
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| NITROGLYCERIN
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PD-Rx Pharmaceuticals, Inc. | 156893695 | repack(43063-918, 43063-916, 43063-919) | |
Revised: 3/2021
Document Id: bd474299-34e2-334b-e053-2995a90a55de
Set id: bbd659a4-b3cb-4da5-ae92-4d0761028c6c
Version: 8
Effective Time: 20210311
PD-Rx Pharmaceuticals, Inc.