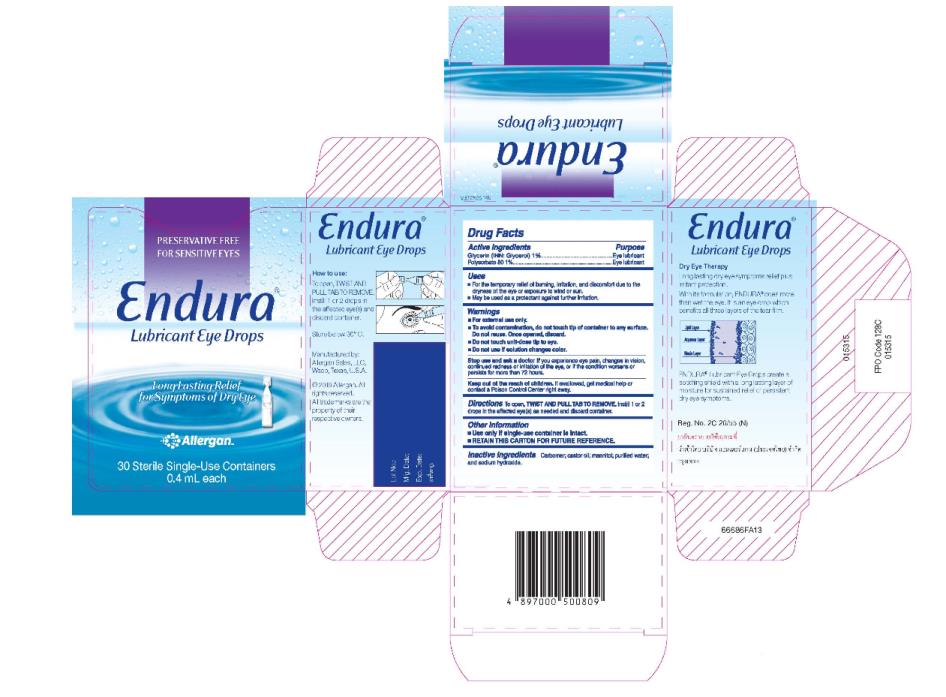
Label: ENDURA- glycerin and polysorbate 80 solution/ drops
- NDC Code(s): 0023-9235-20
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated November 26, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Principal Display Panel - Carton Label
-
INGREDIENTS AND APPEARANCE
ENDURA
glycerin and polysorbate 80 solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0023-9235 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 1 g in 100 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) (POLYSORBATE 80 - UNII:6OZP39ZG8H) POLYSORBATE 80 1 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) CASTOR OIL (UNII: D5340Y2I9G) MANNITOL (UNII: 3OWL53L36A) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0023-9235-20 30 in 1 CARTON 05/14/2012 1 0.4 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/14/2012 Labeler - Allergan, Inc. (144796497)