Label: EXACT-RX SODIUM SULFACETAMIDE AND SULFER 10%/5% CLEANSER- sodium sulfacetamide, sulfur lotion
- NDC Code(s): 42808-113-06, 42808-113-12
- Packager: Exact-Rx, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
DIRECTIONS FOR USE: Wash affected area once or twice daily, or
as directed by your physician. Avoid contat with eyes or mucous
membranes. Wet skin and liberally apply to areas to be cleansed,
massage gently into skin for 10-20 seconds working into a full lather, rinse
thoroughly and pat dry. if drying occurs, it may be controlled by rinsing
cleanser off sooner or using less often. See package insert for complete product information.
- WARNINGS
- WARNINGS
-
CONTRAINDICATIONS
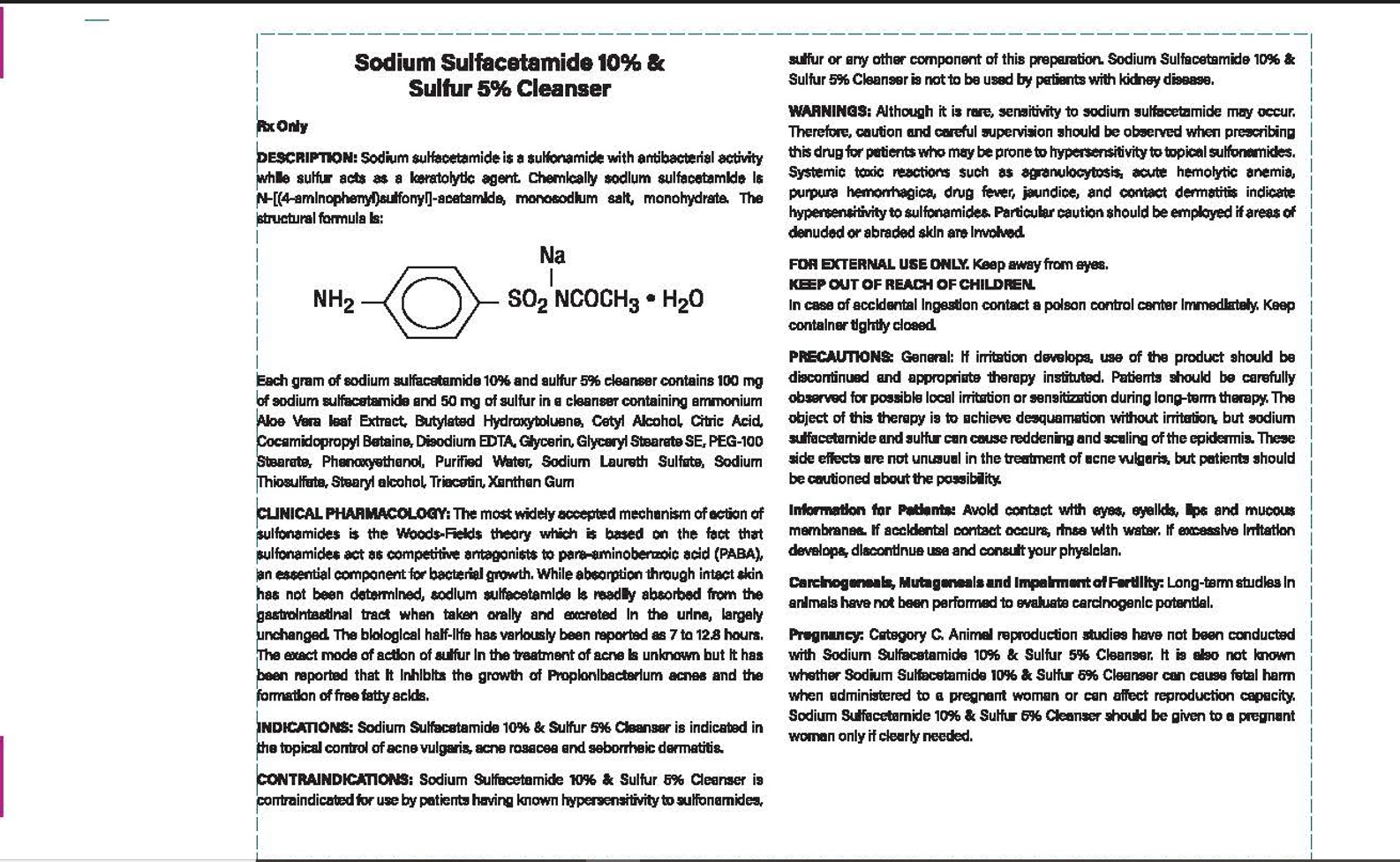
CONTRAINDICATIONS: Sodium Sulfacetamide 10% & Sulfer 5% Cleanser is
contraindicated in persons with know or suspected hypersensitivity to sulfonamides,
sulfer or any other component of this preparation. Sodium Sulfacetamide 10% & Sulfer %5%
Cleanser is not to be used by patients with kidney disease.
CAUTION: If redness or irritaiton occurs, discontinue use.
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- DESCRIPTION
- PATIENT PACKAGE INSERT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXACT-RX SODIUM SULFACETAMIDE AND SULFER 10%/5% CLEANSER
sodium sulfacetamide, sulfur lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42808-113 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFACETAMIDE SODIUM (UNII: 4NRT660KJQ) (SULFACETAMIDE - UNII:4965G3J0F5) SULFACETAMIDE SODIUM 100 mg in 1 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 50 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CETYL ALCOHOL (UNII: 936JST6JCN) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) SODIUM THIOSULFATE (UNII: HX1032V43M) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) TRIACETIN (UNII: XHX3C3X673) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42808-113-06 170 g in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2011 2 NDC:42808-113-12 340 g in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/01/2011 Labeler - Exact-Rx, Inc. (137953498)