CELLZYME ON-TOX- peppermint oil eucalyptus oil ferric oxide red cream
PHARMACAL-INTERNATIONAL. CO., LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
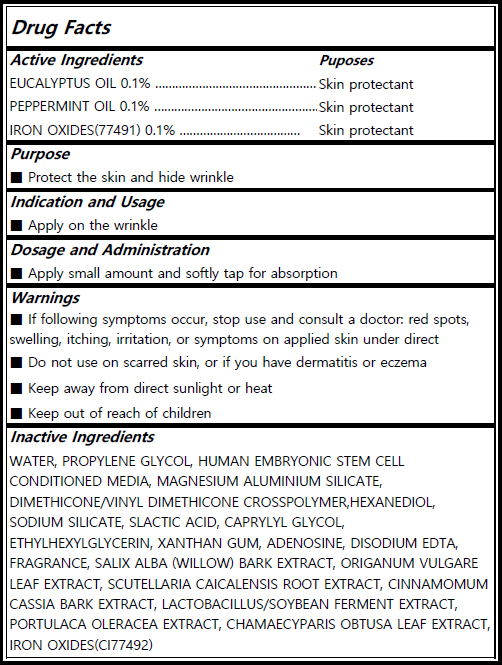
Actvie Ingrdients
IRON OXIDES (skin protectant /0.1%) EUCALYPTUS OIL (skin protectant/0.1%) PEPPERMINT OIL (skin protectant/0.1%)
Inactives
WATER, PROPYLENE GLYCOL, HUMAN EMBRYONIC STEM CELL CONDITIONED MEDIA, MAGNESIUM ALUMINIUM SILICATE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER,HEXANEDIOL, SODIUM SILICATE, SLACTIC ACID, CAPRYLYL GLYCOL, ETHYLHEXYLGLYCERIN, XANTHAN GUM, ADENOSINE, DISODIUM EDTA, FRAGRANCE, SALIX ALBA (WILLOW) BARK EXTRACT, ORIGANUM VULGARE LEAF EXTRACT, SCUTELLARIA CAICALENSIS ROOT EXTRACT, CINNAMOMUM CASSIA BARK EXTRACT, LACTOBACILLUS/SOYBEAN FERMENT EXTRACT, PORTULACA OLERACEA EXTRACT, CHAMAECYPARIS OBTUSA LEAF EXTRACT, IRON OXIDES(CI77492)
Indication and Usage
For hiding wrinkle and skin protectant
Dosage and Administration
Apply small amount and softly tap for absorption.
Warnings
If following symptoms occur, stop use and consult a doctor: red spots, swelling, itching, irritation, or symptoms on applied skin under direct sunlight. Do not use on scarred skin, or if you have dermatitis or eczema. Keep away from direct sunlight or heat. Keep out of reach of children
Keep out of reach of children
Product Labels

PHARMACAL-INTERNATIONAL. CO., LTD

