Label: PROHIBIT SOLUBLE DRENCH POWDER- levamisole hydrochloride powder, for solution
- NDC Code(s): 23243-2320-5, 23243-2320-6
- Packager: Huvepharma, Inc
- Category: OTC ANIMAL DRUG LABEL
Drug Label Information
Updated December 18, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
VETERINARY INDICATIONS
CATTLE AND SHEEP DEWORMER FOR ORAL USE
Administer as a standard drench with standard drench syringe or administer as a concentrated
drench solution with an automatic drenching syringe.INDICATIONS:
Prohibit (levamisole hydrochloride) is a broad-spectrum anthelmintic and is effective against
the following nematode infections in cattle and sheep:SHEEP:
STOMACH WORMS: Haemonchus contortus, Trichostrongylus axei, Teladorsagia circumcincta.
INTESTINAL WORMS: Trichostrongylus colubriformis, Cooperia curticei, Nematodirus spathiger,
Bunostomum trigonocephalum, Oesophagostomum columbianum, Chabertia ovina.
LUNGWORMS: Dictyocaulus filaria.CATTLE:
STOMACH WORMS: Haemonchus placei, Trichostrongylus axei, Ostertagia ostertagi.
INTESTINAL WORMS: Trichostrongylus longlspicularis, Cooperia oncophora, Cooperia punctata,
Nematodirus spathiger, Bunostomum phiebotomum, Oesophagostomum radiatum.
LUNGWORMS: Dictyocaulus viviparus. - GENERAL PRECAUTIONS
-
DOSAGE & ADMINISTRATION
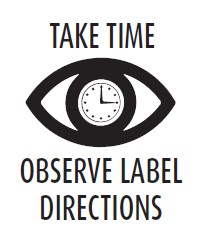
DOSAGE AND ADMINISTRATION
CATTLE–STANDARD DRENCH SOLUTION: Place the contents of this packet in a 1 quart (32 fl. oz.) container, fill with water, swirl until dissolved. Administer as a single drench dose according to the following table:
SHEEP–STANDARD DRENCH SOLUTION: Place the contents of this packet in a 1 gallon (128 fl. oz.) container, fill with water, swirl until dissolved. Administer as a single drench dose according to the following table:
Weight
Drench dosage
Packet Will Treat
Weight
Drench Dosage
Packet Will Treat
200 lb.
1/2 fl. oz.
64 head
50 lb.
1/2 fl. oz.
256 head
400 lb.
1 fl. oz.
32 head
100 lb.
1 fl. oz.
128 head
650 lb.
1 1/2 fl. oz.
21 head
150 lb.
1 1/2 fl. oz.
84 head
800 lb.
2 fl. oz.
16 head
200 lb.
2 fl. oz.
64 head
CONCENTRATED DRENCH SOLUTION: For use with automatic syringe. Place the contents of this packet in a standard household measuring container and add water to the 8 3/4 fl. oz. level; or use the measuring container available from your supplier and add water to the mark. Swirl until dissolved. Give 2 ml (milliliter) per 100 lb. body weight. Refer to the table above for the number of cattle this packet will treat.
CONCENTRATED DRENCH SOLUTION: For use with automatic syringe. Place the contents of this packet in a standard household measuring container and add water to the 17 1/2 fl. oz. level. Swirl until dissolved. Give 2 ml per 50 lb. body weight. Refer to the table above for the number of sheep this packet will treat.
Do not underdose. Ensure each animal receives a complete dose based on a current body weight.
Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.NOTE: Careful weight estimates are essential for proper performance of this product. Prepare solutions
as needed. However, excess solutions may be stored in clean closed containers up to 90 days without
loss of anthelmintic activity. Cattle and Sheep maintained under conditions of constant helminth
exposure may require re-treatment within two to four weeks after the first treatment. - RESIDUE WARNING
-
WARNINGS
OTHER WARNINGS: Parasite resistance may develop to any dewormer, and has been
reported for most classes of dewormers. Treatment with a dewormer used in
conjunction with parasite management practices appropriate to the geographic
area and the animal(s) to be treated may slow the development of parasite
resistance. Fecal examinations or other diagnostic tests and parasite
management history should be used to determine if the product is appropriate
for the herd/flock, prior to the use of any dewormer. Following the use of any
dewormer, effectiveness of treatment should be monitored (for example, with
the use of a fecal egg count reduction test or another appropriate method).
A decrease in a drug's effectiveness over time as calculated by fecal egg
count reduction tests may indicate the development of resistance to the
dewormer administered. Your parasite management plan should be adjusted
accordingly based on regular monitoring. -
GENERAL PRECAUTIONS
CAUTION: Muzzle foam may be observed. However, this reaction will
disappear within a few hours. If this condition persists, a veterinarian
should be consulted. Follow recommended dosage carefully. Consult
veterinarian before using in severely debilitated animals.
Consult your veterinarian for assistance in the diagnosis, treatment,
and control of parasitism. -
ADVERSE REACTIONS
To report suspected adverse drug events, for technical assistance or to
obtain a copy of the Safety Data Sheet (SDS), contact Huvepharma, Inc.
at 1-877-994-4883 or www.huvepharma.us. For additional information about
adverse drug experience reporting for animal drugs, contact FDA at
1-888-FDA-VETS or http://www.fda.gov/reportanimalae. - STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- VETERINARY INDICATIONS
- GENERAL PRECAUTIONS
-
VETERINARY INDICATIONS
INDICATIONS: PROHIBIT (levamisole hydrochloride) is a broad-spectrum anthelmintic and is
effective against the following nematode infections in cattle and sheep:
SHEEP:
STOMACH WORMS: Haemonchus contortus, Trichostrongylus axei, Teladorsagia circumcincta.
INTESTINAL WORMS: Trichostrongylus colubriformis, Cooperia curticei, Nematodirus spathiger,
Bunostomum trigonocephalum, Oesophagostomum columbianum, Chabertia ovina.
LUNGWORMS: Dictyocaulus filaria.
CATTLE:
STOMACH WORMS: Haemonchus placei, Trichostrongylus axei, Ostertagia ostertagi.
INTESTINAL WORMS: Trichostrongylus longlspicularis, Cooperia oncophora, Cooperia punctata,
Nematodirus spathiger, Bunostomum phiebotomum, Oesophagostomum radiatum.
LUNGWORMS: Dictyocaulus viviparus. - RESIDUE WARNING
-
WARNINGS
OTHER WARNINGS: Parasite resistance may develop to any dewormer, and has been reported
for most classes of dewormers. Treatment with a dewormer used in conjunction with parasite
management practices appropriate to the geographic area and the animal(s) to be treated may
slow the development of parasite resistance. Fecal examinations or other diagnostic tests and
parasite management history should be used to determine if the product is appropriate for the
herd/flock, prior to the use of any dewormer. Following the use of any dewormer, effectiveness
of treatment should be monitored (for example, with the use of a fecal egg count reduction
test or another appropriate method). A decrease in a drug's effectiveness over time as
calculated by fecal egg count reduction tests may indicate the development of resistance to
the dewormer administered. Your parasite management plan should be adjusted accordingly
based on regular monitoring. -
GENERAL PRECAUTIONS
CAUTION: Muzzle foam may be observed. However, this reaction will disappear within a few
hours. If this condition persists, a veterinarian should be consulted. Follow recommended
dosage carefully. Consult veterinarian before using in severely debilitated animals.Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
- STORAGE AND HANDLING
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: When you are ready to deworm your cattle or sheep, add water to the powder in this bottle up to the 3 liter mark. Swirl to mix thoroughly before using. If any solution is left over, it may be stored for up to 3 months in this tightly capped bottle, shake well before using.
DATE WATER WAS ADDED TO THIS BOTTLE
Month Day Year
Administer as a single drench dose as follows:
CATTLE--2 mL per 100 lb. body weight
Weight
Drench dosage
Bottle
Will Treat
100 lb.
2 mL
1,500 head
300 lb.
6 mL
500 head
500 lb.
10 mL
300 head
700 lb.
14 mL
214 head
1,000 lb.
20 mL
150 head
SHEEP--1 mL per 50 lb. body weight
Weight
Drench dosage
Bottle
Will Treat
50 lb.
1 mL
3,000 head
100 lb.
2 mL
1,500 head
150 lb.
3 mL
1,000 head
200 lb.
4 mL
750 head
Do not underdose. Ensure each animal receives a complete dose based on a current body weight.
Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.NOTE: Careful weight estimates are essential for proper performance of this
product. Cattle and sheep maintained under conditions of constant helminth
exposure may require retreatment within two to four weeks after the first
treatment. -
ADVERSE REACTIONS
To report suspected adverse drug events, for technical assistance or to
obtain a copy of the Safety Data Sheet (SDS), contact Huvepharma, Inc. at
1-877-994-4883 or www.huvepharma.us. For additional information about
adverse drug experience reporting for animal drugs, contact FDA at
1-888-FDA-VETS or http://www.fda.gov/reportanimalae. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROHIBIT SOLUBLE DRENCH POWDER
levamisole hydrochloride powder, for solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:23243-2320 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVAMISOLE HYDROCHLORIDE (UNII: DL9055K809) (LEVAMISOLE - UNII:2880D3468G) LEVAMISOLE 0.9 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-2320-5 52 g in 1 PACKET 2 NDC:23243-2320-6 605 g in 1 JUG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200225 04/20/2022 Labeler - Huvepharma, Inc (619153559) Registrant - Huvepharma EOOD (552671651)