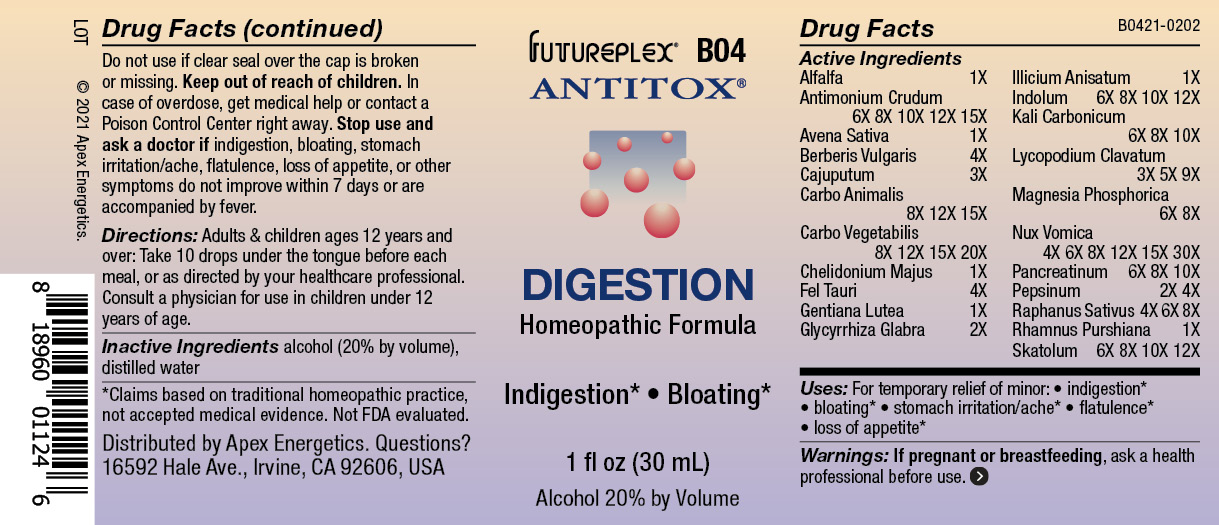
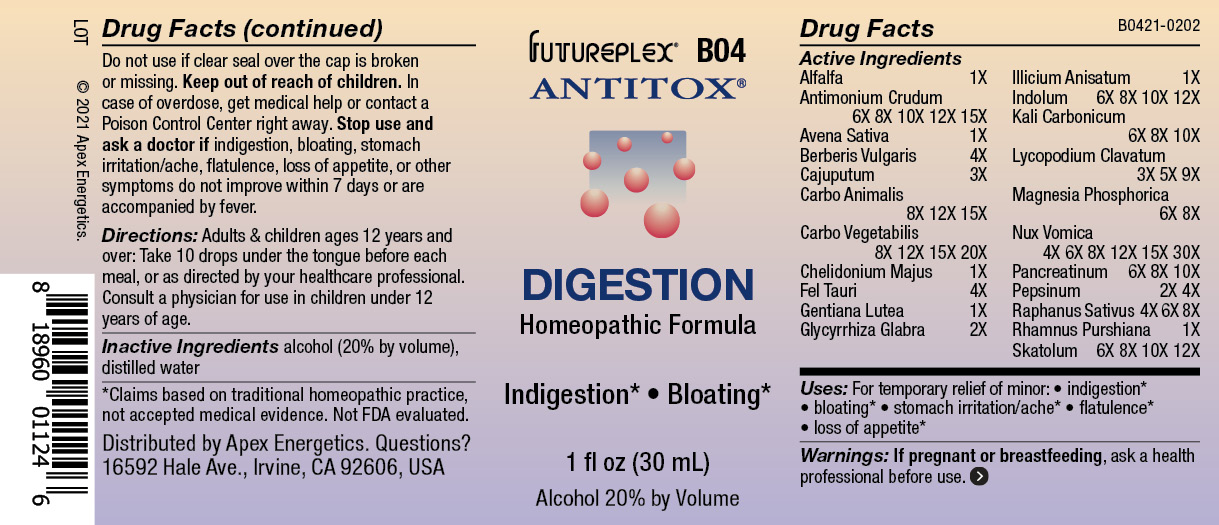
Label: B04 DIGESTION- alfalfa, antimonium crudum, avena sativa, berberis vulgaris, cajuputum, carbo animalis, carbo vegetabilis, chelidonium majus, fel tauri, gentiana lutea, glycyrrhiza glabra, illicium anisatum, indolum, kali carbonicum, lycopodium clavatum, magnesia phosphorica, nux vomica, pancreatinum, pepsinum, raphanus sativus, rhamnus purshiana, skatolum solution/ drops
- NDC Code(s): 63479-0204-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Alfalfa
1X
Antimonium Crudum
6X 8X 10X 12X 15X
Avena Sativa
1X
Berberis Vulgaris
4X
Cajuputum
3X
Carbo Animalis
8X 12X 15X
Carbo Vegetabilis
8X 12X 15X 20X
Chelidonium Majus
1X
Fel Tauri
4X
Gentiana Lutea
1X
Glycyrrhiza Glabra
2X
Illicium Anisatum
1X
Indolum
6X 8X 10X 12X
Kali Carbonicum
6X 8X 10X
Lycopodium Clavatum
3X 5X 9X
Magnesia Phosphorica
6X 8X
Nux Vomica
4X 6X 8X 12X 15X 30X
Pancreatinum
6X 8X 10X
Pepsinum
2X 4X
Raphanus Sativus
4X 6X 8X
Rhamnus Purshiana
1X
Skatolum
6X 8X 10X 12X
- INDICATIONS & USAGE
- WARNINGS
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
B04 DIGESTION
alfalfa, antimonium crudum, avena sativa, berberis vulgaris, cajuputum, carbo animalis, carbo vegetabilis, chelidonium majus, fel tauri, gentiana lutea, glycyrrhiza glabra, illicium anisatum, indolum, kali carbonicum, lycopodium clavatum, magnesia phosphorica, nux vomica, pancreatinum, pepsinum, raphanus sativus, rhamnus purshiana, skatolum solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-0204 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE (UNII: FQ3DRG0N5K) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 10 [hp_X] in 1 mL PEPSIN (UNII: GID333S43J) (PEPSIN - UNII:GID333S43J) PEPSIN 4 [hp_X] in 1 mL FRANGULA PURSHIANA BARK (UNII: 4VBP01X99F) (FRANGULA PURSHIANA BARK - UNII:4VBP01X99F) FRANGULA PURSHIANA BARK 1 [hp_X] in 1 mL GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) (GLYCYRRHIZA GLABRA - UNII:2788Z9758H) GLYCYRRHIZA GLABRA 2 [hp_X] in 1 mL STAR ANISE (UNII: XKC1657P78) (STAR ANISE - UNII:XKC1657P78) STAR ANISE 1 [hp_X] in 1 mL POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 10 [hp_X] in 1 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 20 [hp_X] in 1 mL BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 4 [hp_X] in 1 mL CAJUPUT OIL (UNII: J3TO6BUQ37) (CAJUPUT OIL - UNII:J3TO6BUQ37) CAJUPUT OIL 3 [hp_X] in 1 mL CARBO ANIMALIS (UNII: 279O8I0433) (CARBO ANIMALIS - UNII:279O8I0433) CARBO ANIMALIS 15 [hp_X] in 1 mL BOS TAURUS BILE (UNII: ET3651ZLOU) (BOS TAURUS BILE - UNII:ET3651ZLOU) BOS TAURUS BILE 4 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 9 [hp_X] in 1 mL MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 8 [hp_X] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_X] in 1 mL GENTIANA LUTEA ROOT (UNII: S72O3284MS) (GENTIANA LUTEA ROOT - UNII:S72O3284MS) GENTIANA LUTEA ROOT 1 [hp_X] in 1 mL INDOLE (UNII: 8724FJW4M5) (INDOLE - UNII:8724FJW4M5) INDOLE 12 [hp_X] in 1 mL SKATOLE (UNII: 9W945B5H7R) (SKATOLE - UNII:9W945B5H7R) SKATOLE 12 [hp_X] in 1 mL AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 1 [hp_X] in 1 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 1 [hp_X] in 1 mL RADISH (UNII: EM5RP35463) (RADISH - UNII:EM5RP35463) RADISH 8 [hp_X] in 1 mL ALFALFA (UNII: DJO934BRBD) (MEDICAGO SATIVA WHOLE - UNII:DJO934BRBD) ALFALFA 1 [hp_X] in 1 mL ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 15 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-0204-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 12/15/1994 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/15/1994 Labeler - Apex Energetics Inc. (195816384)