Label: SUCCINYLCHOLINE CHLORIDE injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 72785-0010-1, 72785-0010-7 - Packager: Liva Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 14, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
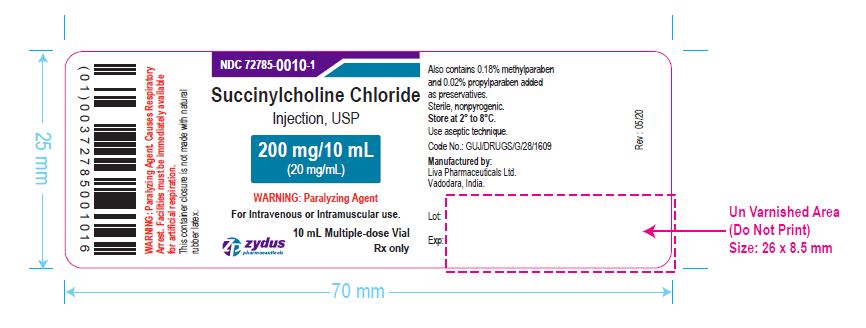
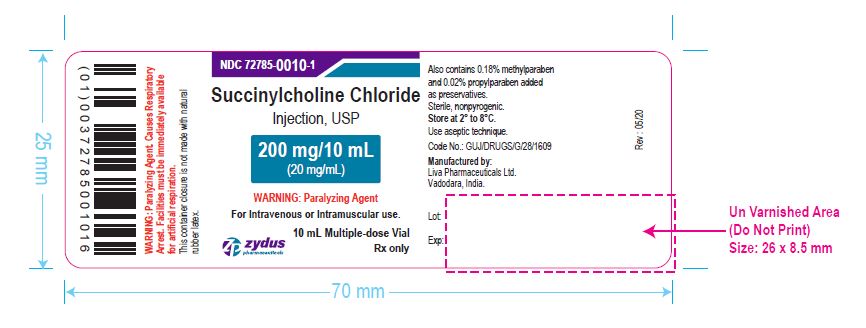
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 72785-0010-1
Succinylcholine Chloride Injection, USP
200 mg/10 mL (20 mg/mL)
For Intravenous or Intramuscular use.
10 mL Multiple-dose Vial
Rx only
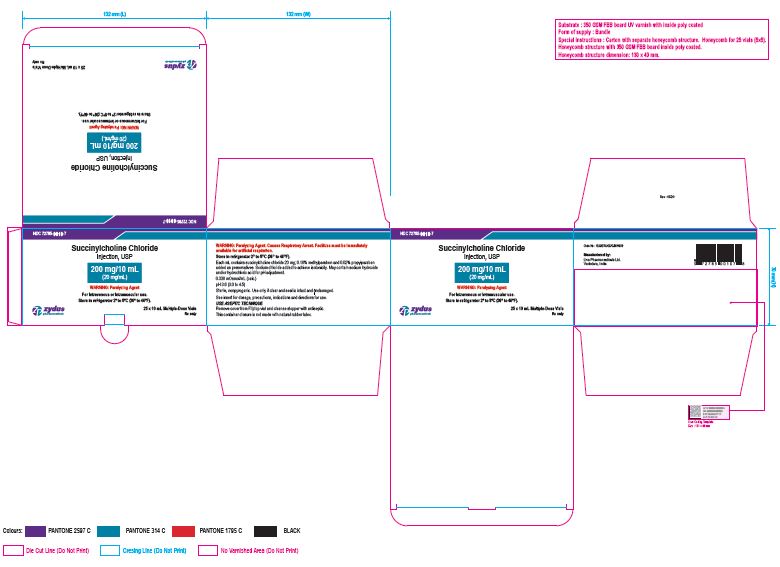
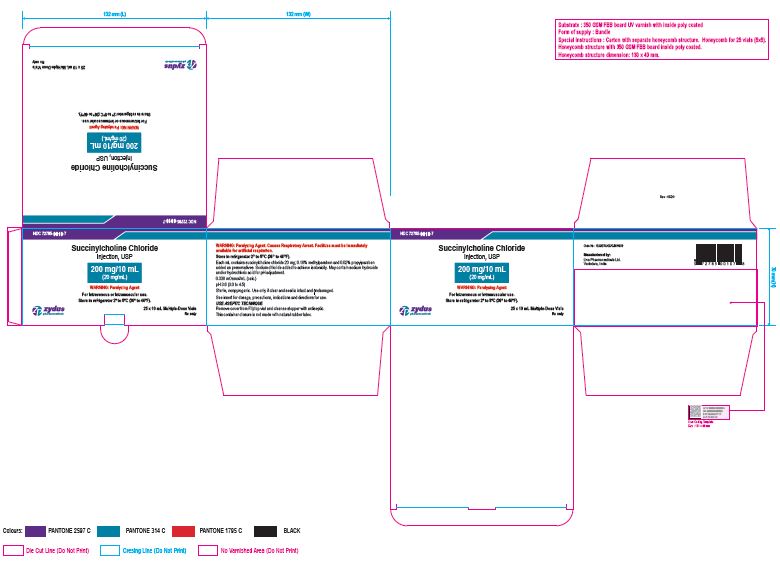
NDC 72785-0010-7
Succinylcholine Chloride Injection, USP
200 mg/10 mL (20 mg/mL)
For Intravenous or Intramuscular use.
Store in refrigerator 2º to 8ºC (36º to 46ºF).
25 x 10 mL Multiple-dose Vial
Rx only
-
INGREDIENTS AND APPEARANCE
SUCCINYLCHOLINE CHLORIDE
succinylcholine chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72785-0010 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUCCINYLCHOLINE CHLORIDE (UNII: I9L0DDD30I) (SUCCINYLCHOLINE - UNII:J2R869A8YF) SUCCINYLCHOLINE CHLORIDE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) METHYLPARABEN (UNII: A2I8C7HI9T) 1.8 mg in 1 mL PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.2 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72785-0010-7 25 in 1 TRAY 05/10/2018 1 NDC:72785-0010-1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209467 05/10/2018 Labeler - Liva Pharmaceuticals Limited (873671928) Registrant - Cadila Healthcare Limited (918596198) Establishment Name Address ID/FEI Business Operations Liva Pharmaceuticals Limited 873671928 MANUFACTURE(72785-0010) , ANALYSIS(72785-0010)