ALOCANE- lidocaine hydrochloride and hydrocortisone acetate ointment
Quest Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Alocane ®
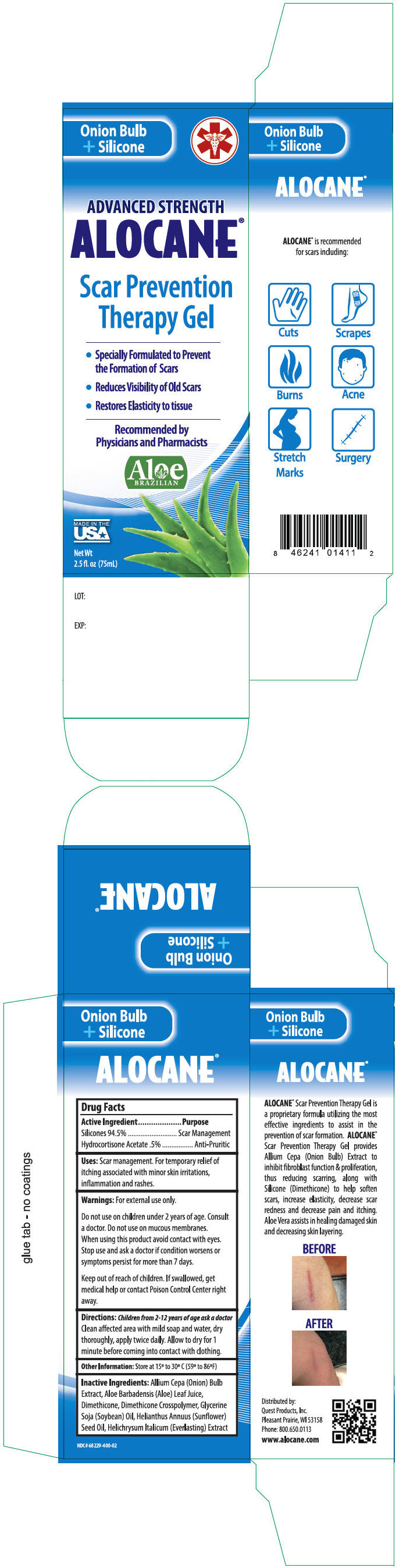
Uses
Scar management. For temporary relief of itching associated with minor skin irritations, inflammation and rashes.
Directions
Children from 2-12 years of age ask a doctor
Clean affected area with mild soap and water, dry thoroughly, apply twice daily. Allow to dry for 1 minute before coming into contact with clothing.
Inactive Ingredients
Allium Cepa (Onion) Bulb Extract, Aloe Barbadensis (Aloe) Leaf Juice, Dimethicone, Dimethicone Crosspolymer, Glycerine Soja (Soybean) Oil, Helianthus Annuus (Sunflower) Seed Oil, Helichrysum Italicum (Everlasting) Extract
PRINCIPAL DISPLAY PANEL - 75 mL Tube Carton
Onion Bulb
Silicone
+
ADVANCED STRENGTH
ALOCANE
®
Scar Prevention
Therapy Gel
-
Specially Formulated to Prevent the
Formation of Scars - Reduces Visibility of Old Scars
- Restores Elasticity to tissue
- Smooths and Softens Skin
Recommended by
Physicians and Pharmacists
MADE IN THE
USA
Net Wt
2.5 fl. oz (75ml)
| ALOCANE
lidocaine hydrochloride and hydrocortisone acetate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Quest Products, Inc. (075402441) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fill Tech USA | 965596435 | manufacture(68229-400) | |