PEDI-BORO SOAK PAKS- aluminum sulfate tetradecahydrate, calcium acetate monohydrate powder, for solution
Pedinol Pharmacal, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Pedi-Boro® Soak Paks
| Active Ingredient (in each packet) | Purpose |
|---|---|
|
Aluminum sulfate tetradecahydrate, 1191 mg |
Astringent* |
|
Calcium acetate monohydrate, 839 mg |
Astringent* |
|
*When combined together in water, these ingredients form the active ingredient aluminum acetate. See Directions. |
|
Uses
Temporarily relieves minor skin irritations due to:
- •
- poison ivy
- •
- poison oak
- •
- poison sumac
- •
- insect bites
- •
- athlete's foot
- •
- rashes caused by soaps, detergents, cosmetics or jewelry
Warnings
For external use only
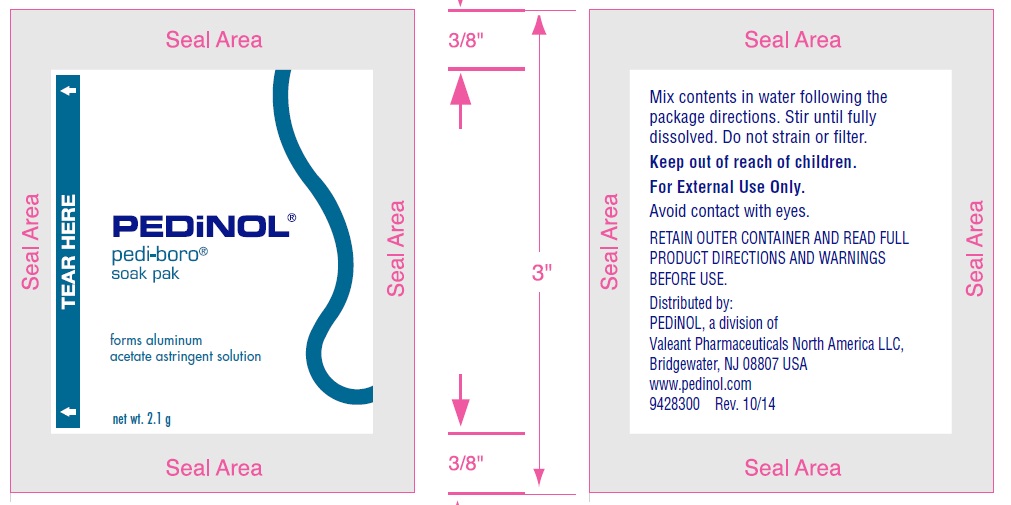
Directions
- •
- dissolve 1 to 3 packets in a pint (16 oz) of cool or warm water
- •
- stir until fully dissolved; do not strain or filter. The resulting mixture contains 0.13% (1 packet), 0.26% (2 packets), or 0.41% (3 packets) aluminum acetate and is ready for use.
For use as a soak:
- •
- soak affected area for 15 to 30 minutes as needed, or as directed by a doctor
- •
- repeat 3 times a day or as directed by a doctor
- •
- discard solution after each use
For use as a compress or wet dressing:
- •
- soak a clean, soft cloth in the solution
- •
- apply cloth loosely to affected area for 15 to 30 minutes
- •
- repeat as needed or as directed by a doctor
- •
- discard solution after each use
Questions or comments?
Please call 1-800-321-4576 or visit us at www.pedinaol.com
Retain this packaging for full directions and warnings.
| PEDI-BORO SOAK PAKS
aluminum sulfate tetradecahydrate, calcium acetate monohydrate powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pedinol Pharmacal, Inc. (064737125) |
| Registrant - Valeant Pharmaceuticals North America LLC (042230623) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ULTRAtab Laboratories, Inc. | 151051757 | MANUFACTURE(0884-1706) | |