AK47 MENTHOL COOLING- menthol cream
CROSSTOWN CONCEPTS CORPORATION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
AK4 7 Blue Menthol Cream
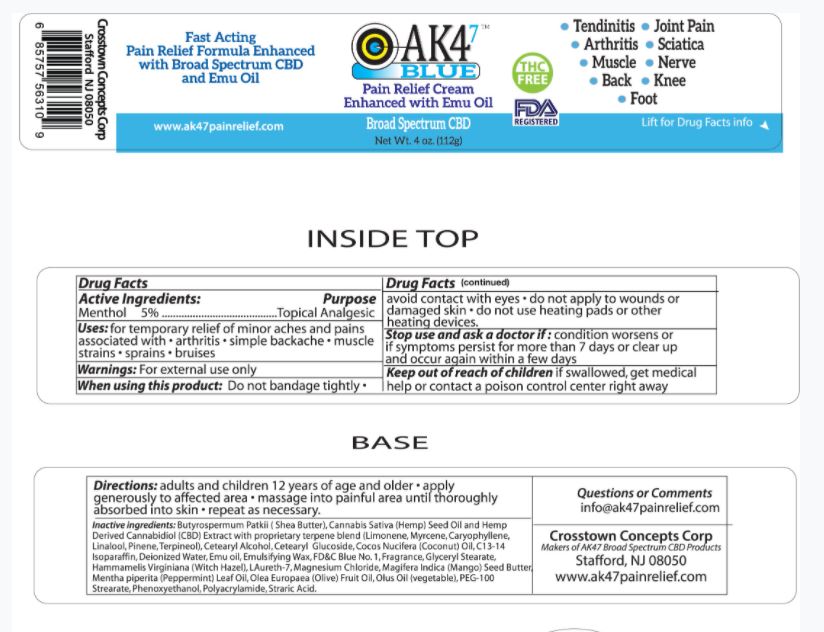
Stop use and ask a doctor if: condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of reach of children if swallowed, get medical help or contact a poison control center right away
When using this product: Do not bandage tightly
- avoid contact with eyes
- do not apply to wounds or damaged skin
- do not use heating pads or other heating devices.
Uses: for temporary relief of minor aches and pains associated with
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
Directions
adults and children 12 years of age and older
- apply generously to affected area
- massage into painful area until thoroughy absorbed into skin
- repeat as necessary.
Butyrospermum Parkii (Shea Butter), Cannabis Sativa (Hemp) Seed Oil and Hemp Derived Cannabidiol (CBD) Extract with proprietary terpene blend (Limonene, Myrcene, Caryophyllene, Linalool, Pinene, Terpineol), Cetearyl Alcohol, Cetearyl Glucoside, Cocos Nucifera (Coconut) Oil, C13-14 Isoparaffin, Deionized Water, Emulsifying Wax, Fragrance, Glyceryl Stearate, Hamamelis Virginiana (Witch Hazel), Laureth-7, Magnesium Chloride, Mangifera Indica (Mango) Seed Butter, Mentha piperita (Peppermint) Leaf Oil, Olea Europaea (Olive) Fruit Oil, Olus Oil (vegetable), PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Stearic Acid.
| AK47 MENTHOL COOLING
menthol cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CROSSTOWN CONCEPTS CORPORATION (009543155) |
| Registrant - RENU LABORATORIES, INC. (945739449) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| RENU LABORATORIES, INC. | 945739449 | manufacture(90078-577) | |