Label: D T B T- calcium tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 81445-0009-1 - Packager: coexleaders co.,ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 4, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
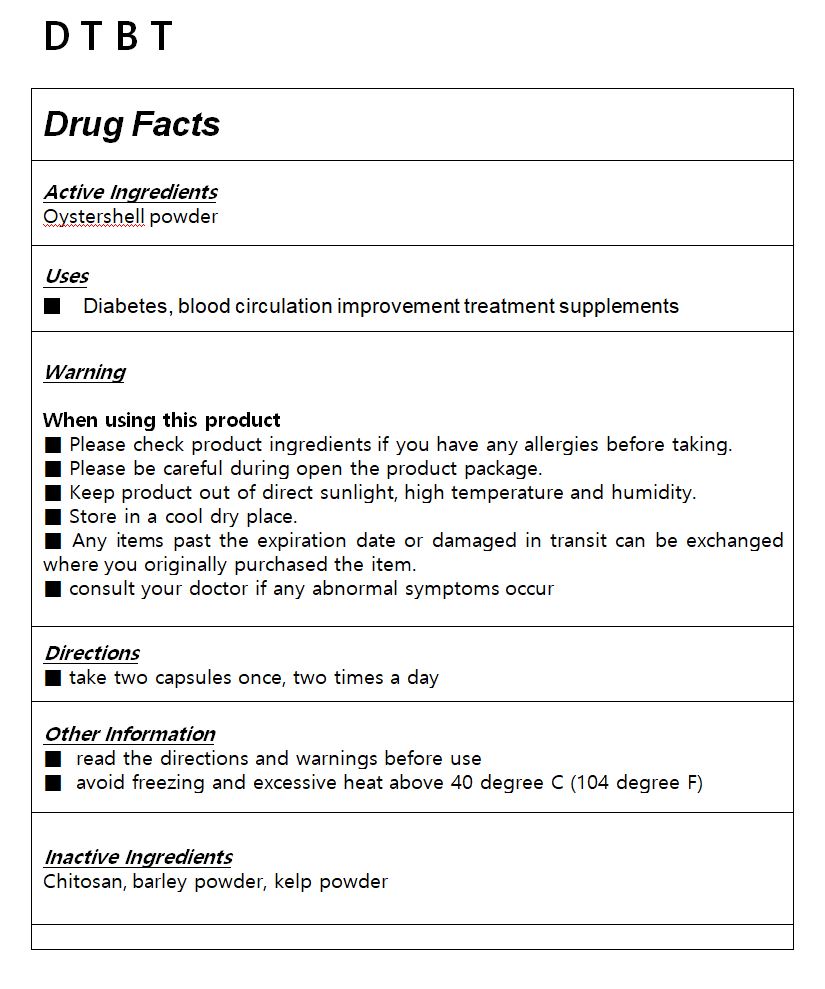
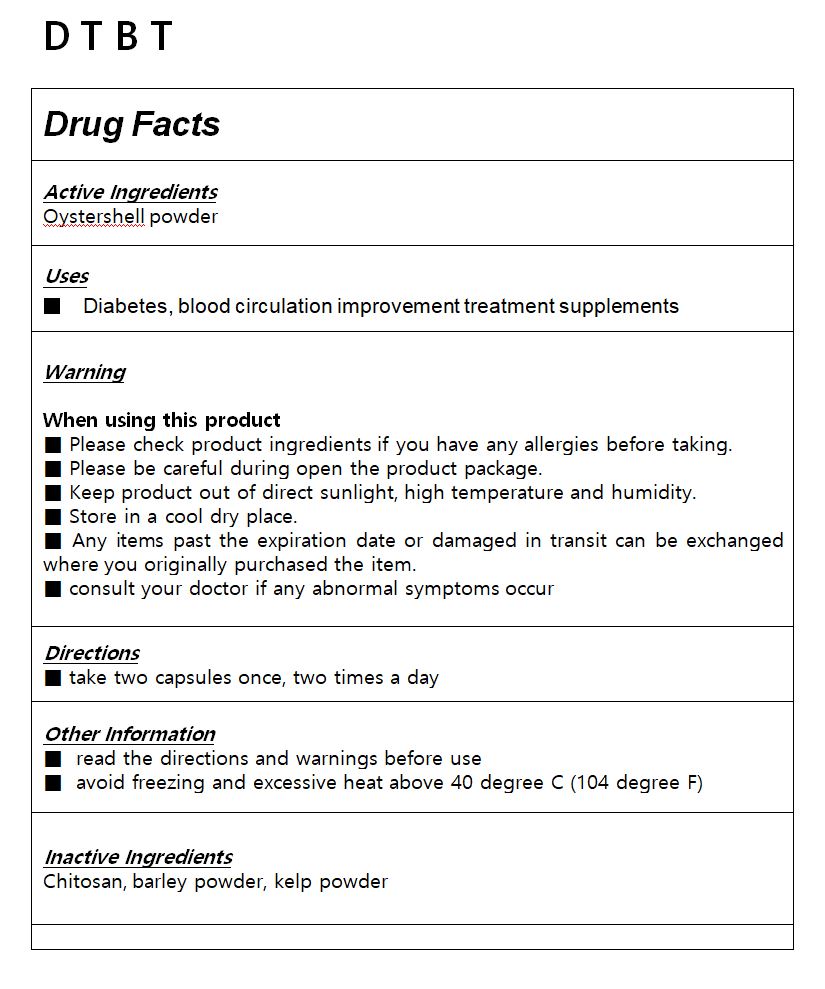
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
WARNING
Please check product ingredients if you have any allergies before taking.
Please be careful during open the product package.
Keep product out of direct sunlight, high temperature and humidity.
Store in a cool dry place.
Any items past the expiration date or damaged in transit can be exchanged where you originally purchased the item.
consult your doctor if any abnormal symptoms occur
- USES
- INDICATION & USAGE SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
D T B T
calcium tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81445-0009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM (UNII: SY7Q814VUP) (CALCIUM - UNII:SY7Q814VUP) CALCIUM 0.08 Inactive Ingredients Ingredient Name Strength CHITOSAN OLIGOSACCHARIDE (UNII: 23R93M6Y64) Product Characteristics Color white Score score with uneven pieces Shape OVAL Size 12mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81445-0009-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/25/2021 Labeler - coexleaders co.,ltd. (695097730) Registrant - coexleaders co.,ltd. (695097730) Establishment Name Address ID/FEI Business Operations coexleaders co.,ltd. 695097730 manufacture(81445-0009)