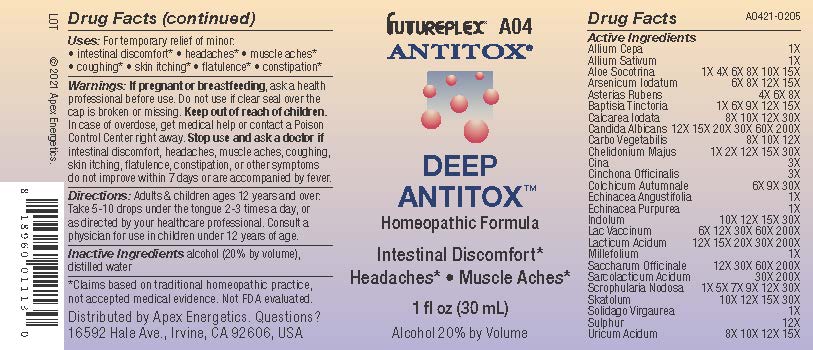
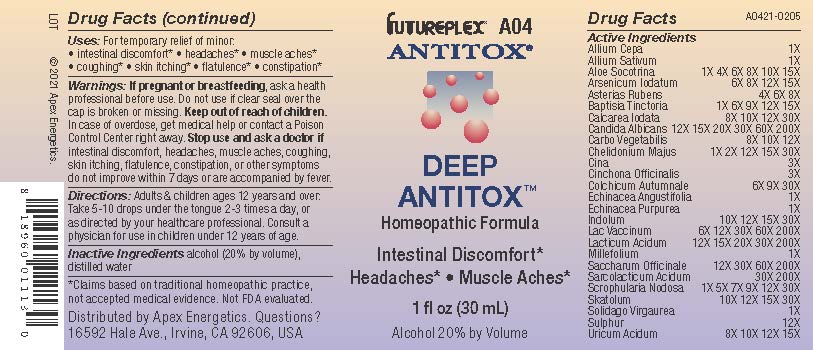
Label: A04 DEEP ANTITOX- allium cepa, allium sativum, aloe socotrina, arsenicum iodatum, asterias rubens, baptisia tinctoria, calcarea iodata, candida albicans, carbo vegetabilis, chelidonium majus, cina, cinchona officinalis, colchicum autumnale, echinacea angustifolia, echinacea purpurea, indolum, lac vaccinum, lacticum acidum, millefolium, saccharum officinale, sarcolacticum acidum, scrophularia nodosa, skatolum, solidago virgaurea, sulphur, uricum acidum solution/ drops
- NDC Code(s): 63479-0104-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Allium Cepa
1X
Allium Sativum
1X
Aloe Socotrina
1X 4X 6X 8X 10X 15X
Arsenicum Iodatum
6X 8X 12X 15X
Asterias Rubens
4X 6X 8X
Baptisia Tinctoria
1X 6X 9X 12X 15X
Calcarea Iodata
8X 10X 12X 30X
Candida Albicans
12X 15X 20X 30X 60X 200X
Carbo Vegetabilis
8X 10X 12X
Chelidonium Majus
1X 2X 12X 15X 30X
Cina
3X
Cinchona Officinalis
3X
Colchicum Autumnale
6X 9X 30X
Echinacea Angustifolia
1X
Echinacea Purpurea
1X
Indolum
10X 12X 15X 30X
Lac Vaccinum
6X 12X 30X 60X 200X
Lacticum Acidum
12X 15X 20X 30X 200X
Millefolium
1X
Saccharum Officinale
12X 30X 60X 200X
Sarcolacticum Acidum
30X 200X
Scrophularia Nodosa
1X 5X 7X 9X 12X 30X
Skatolum
10X 12X 15X 30X
Solidago Virgaurea
1X
Sulphur
12X
Uricum Acidum
8X 10X 12X 15X
- INDICATIONS & USAGE
- Warnings:
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
A04 DEEP ANTITOX
allium cepa, allium sativum, aloe socotrina, arsenicum iodatum, asterias rubens, baptisia tinctoria, calcarea iodata, candida albicans, carbo vegetabilis, chelidonium majus, cina, cinchona officinalis, colchicum autumnale, echinacea angustifolia, echinacea purpurea, indolum, lac vaccinum, lacticum acidum, millefolium, saccharum officinale, sarcolacticum acidum, scrophularia nodosa, skatolum, solidago virgaurea, sulphur, uricum acidum solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-0104 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 1 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA WHOLE (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA WHOLE - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA WHOLE 1 [hp_X] in 1 mL SUCROSE (UNII: C151H8M554) (SUCROSE - UNII:C151H8M554) SUCROSE 200 [hp_X] in 1 mL SCROPHULARIA NODOSA (UNII: 7H443NUB2T) (SCROPHULARIA NODOSA - UNII:7H443NUB2T) SCROPHULARIA NODOSA 30 [hp_X] in 1 mL CANDIDA ALBICANS (UNII: 4D7G21HDBC) (CANDIDA ALBICANS - UNII:4D7G21HDBC) CANDIDA ALBICANS 200 [hp_X] in 1 mL CALCIUM IODIDE (UNII: 8EKI9QEE2H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM IODIDE 30 [hp_X] in 1 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 12 [hp_X] in 1 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 30 [hp_X] in 1 mL ARTEMISIA CINA PRE-FLOWERING TOP (UNII: 28M1820ACT) (ARTEMISIA CINA PRE-FLOWERING TOP - UNII:28M1820ACT) ARTEMISIA CINA PRE-FLOWERING TOP 3 [hp_X] in 1 mL COLCHICUM AUTUMNALE BULB (UNII: 993QHL78E6) (COLCHICUM AUTUMNALE BULB - UNII:993QHL78E6) COLCHICUM AUTUMNALE BULB 30 [hp_X] in 1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 1 [hp_X] in 1 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 1 [hp_X] in 1 mL COW MILK (UNII: 917J3173FT) (COW MILK - UNII:917J3173FT) COW MILK 200 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL URIC ACID (UNII: 268B43MJ25) (URIC ACID - UNII:268B43MJ25) URIC ACID 15 [hp_X] in 1 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 3 [hp_X] in 1 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 15 [hp_X] in 1 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 1 [hp_X] in 1 mL GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 1 [hp_X] in 1 mL ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 15 [hp_X] in 1 mL ARSENIC TRIIODIDE (UNII: 3029988O2T) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIIODIDE 15 [hp_X] in 1 mL ASTERIAS RUBENS (UNII: A7FYY9Q742) (ASTERIAS RUBENS - UNII:A7FYY9Q742) ASTERIAS RUBENS 8 [hp_X] in 1 mL INDOLE (UNII: 8724FJW4M5) (INDOLE - UNII:8724FJW4M5) INDOLE 30 [hp_X] in 1 mL LACTIC ACID, DL- (UNII: 3B8D35Y7S4) (LACTIC ACID, DL- - UNII:3B8D35Y7S4) LACTIC ACID, DL- 200 [hp_X] in 1 mL LACTIC ACID, L- (UNII: F9S9FFU82N) (LACTIC ACID, L- - UNII:F9S9FFU82N) LACTIC ACID, L- 200 [hp_X] in 1 mL SKATOLE (UNII: 9W945B5H7R) (SKATOLE - UNII:9W945B5H7R) SKATOLE 30 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-0104-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 12/15/1994 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/15/1994 Labeler - Apex Energetics Inc. (195816384)