CHAPSTICK LIPSHIELD 365- avobenzone, dimethicone, homosalate, octinoxate, octisalate, oxybenzone stick
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
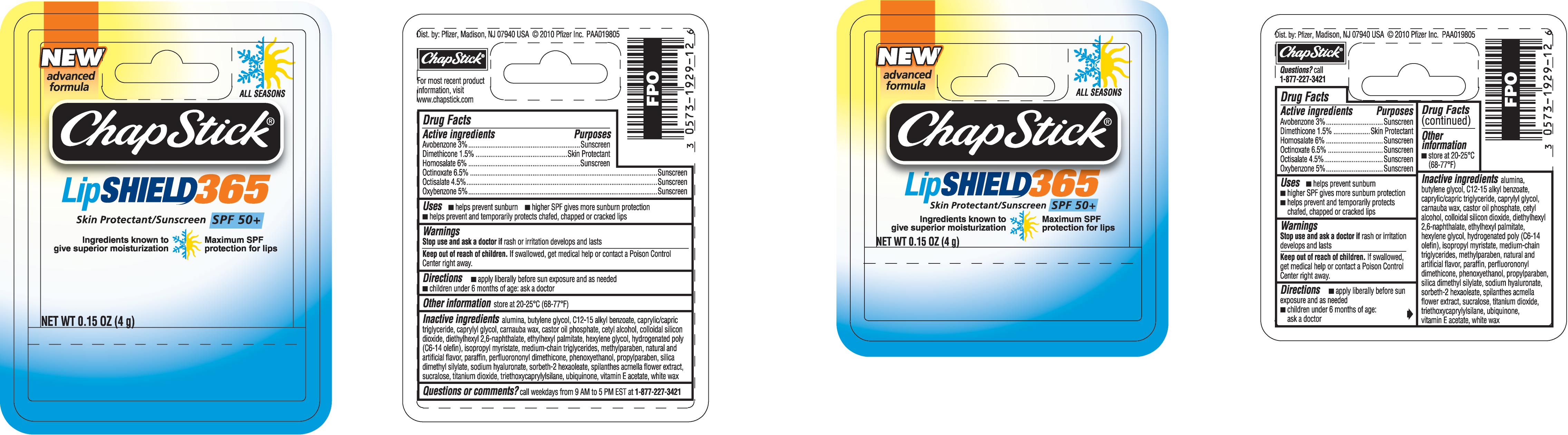
Chapstick LipShield 365
(avobenzone, dimethicone, homosalate, octinoxate, octisalate, and oxybenzone)
ACTIVE INGREDIENTS
Avobenzone 3%
Dimethicone 1.5%
Homosalate 6%
Octinoxate 6.5%
Octisalate 4.5%
Oxybenzone 5%
USES
- •
- helps prevent sunburn
- •
- higher SPF gives more sunburn protection
- •
- helps prevent and temporarily protects chafed, chapped or cracked lips
DIRECTIONS
- •
- apply liberally before sun exposure and as needed
- •
- children under 6 months of age: ask a doctor
INACTIVE INGREDIENTS
alumina, butylene glycol, C12-15 alkyl benzoate, caprylic/capric triglyceride, caprylyl glycol, carnauba wax, castor oil phosphate, cetyl alcohol, colloidal silicon dioxide, diethylhexyl 2,6-naphthalate, ethylhexyl palmitate, hexylene glycol, hydrogenated poly (C6-14 olefin), isopropyl myristate, medium-chain triglycerides, methylparaben, natural and artificial flavor, paraffin, perfluorononyl dimethicone, phenoxyethanol, propylparaben, silica dimethyl silyate, sodium hyaluronate, sorbeth-2 hexaoleate, spilanthes acmella flower extract, sucralose, titanium dioxide, triethoxycaprylysilane, ubiquinone, vitamin E acetate, white wax
PRODUCT PACKAGING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
Chapstick LipShield 365
Skin Protectant/Sunscreen SPF 50+
Ingredients known to give superior moisturization
Maximum SPF protection for lips
All Seasons
New advanced formula
NET WT 0.15 OZ (4 g)
Dist. by: Pfizer, Madison, NJ 07940 USA © 2010 Pfizer Inc.
For most recent product information, visit www.chapstick.com
Sealed For Your Protection
Twist Cap To Break Seal
| CHAPSTICK LIPSHIELD 365
avobenzone, dimethicone, homosalate, octinoxate, octisalate, oxybenzone stick |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |