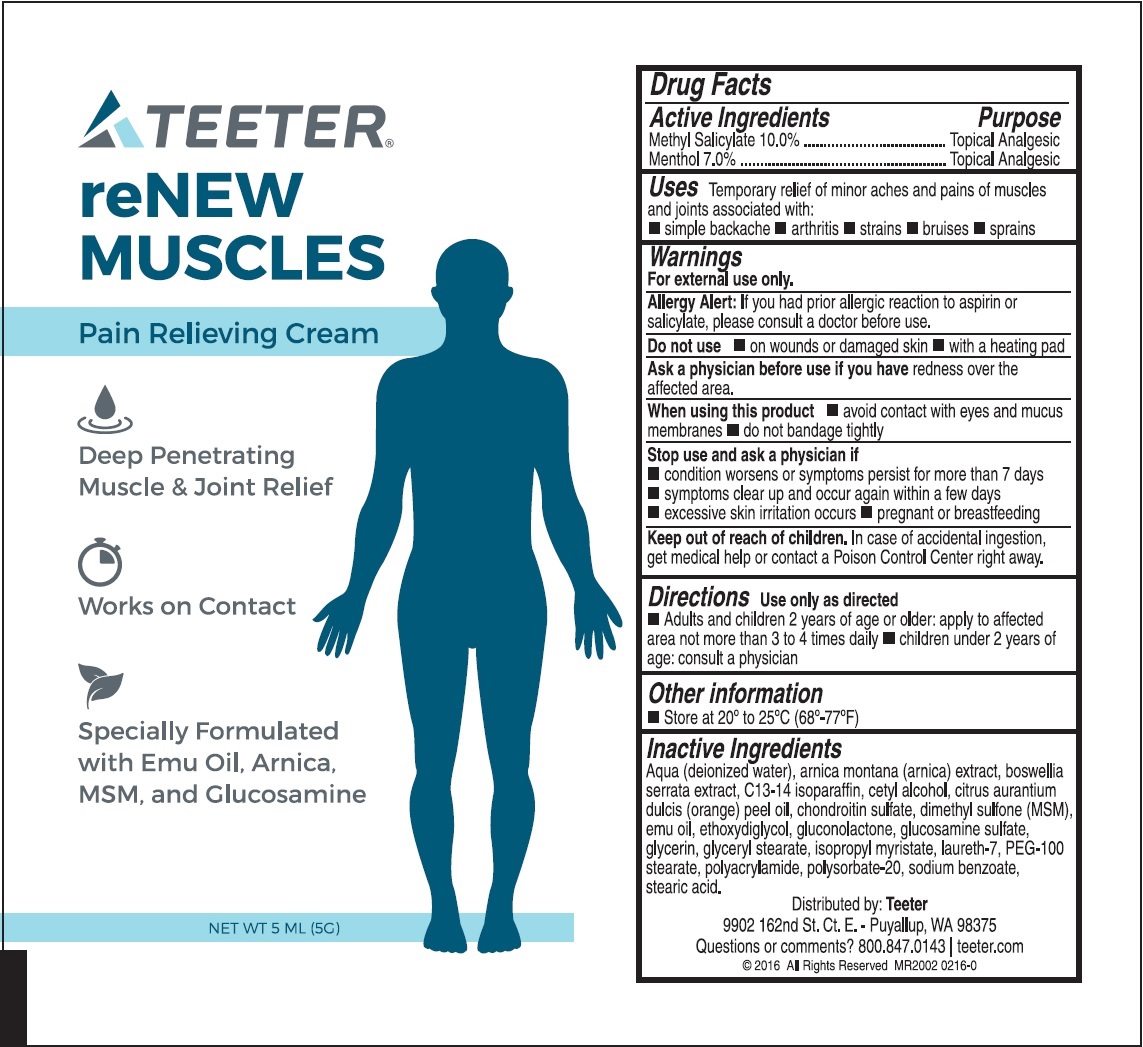
Label: TEETER RENEW MUSCLES PAIN RELIEVING- methyl salicylate, menthol cream
- NDC Code(s): 70329-730-01
- Packager: STL International INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
-
Warnings
For external use only.
Allergy Alert:
If you had prior allergic reaction to aspirin or salicylate, please consult a doctor before use.
- Directions
- Other information
-
Inactive Ingredients
Aqua (deionized water), arnica montana (arnica) extract, boswellia serrata extract, C13-14 isoparaffin, cetyl alcohol, citrus aurantium dulcis (orange) peel oil, chondroitin sulfate, dimethyl sulfone (MSM), emu oil, ethoxydiglycol, gluconolactone, glucosamine sulfate, glycerin, glyceryl stearate, isopropyl myristate, laureth-7, PEG-100 stearate, polyacrylamide, polysorbate-20, sodium benzoate, stearic acid.
- Package Labeling
-
INGREDIENTS AND APPEARANCE
TEETER RENEW MUSCLES PAIN RELIEVING
methyl salicylate, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70329-730 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 100 mg in 1 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 70 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ARNICA MONTANA (UNII: O80TY208ZW) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) CETYL ALCOHOL (UNII: 936JST6JCN) ORANGE OIL (UNII: AKN3KSD11B) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) EMU OIL (UNII: 344821WD61) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LAURETH-7 (UNII: Z95S6G8201) PEG-100 STEARATE (UNII: YD01N1999R) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM BENZOATE (UNII: OJ245FE5EU) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70329-730-01 1 in 1 BOX 02/09/2017 1 5 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/12/2016 Labeler - STL International INC (062729769)