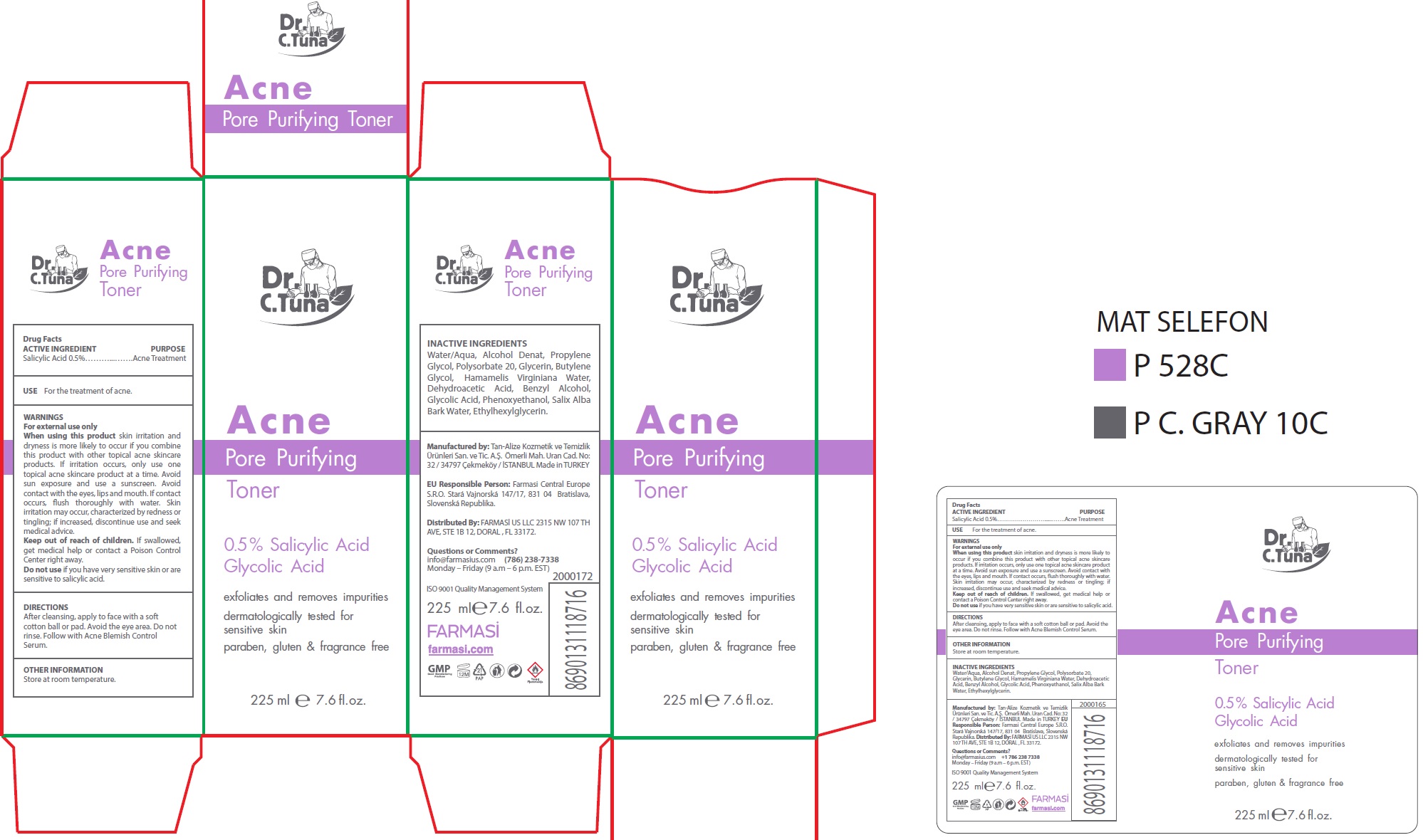
Label: DR. C. TUNA ACNE PORE PURIFYING TONER- salicylic acid liquid
- NDC Code(s): 74690-009-01, 74690-009-02
- Packager: Farmasi US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- USE
-
WARNINGS
For external use only
When using this product
skin irritation and dryness is more likely to occur if you combine this product with other topical acne skincare products. If irritation occurs, only use one topical acne skincare product at a time. Avoid sun exposure and use a sunscreen. Avoid contact with the eyes, lips and mouth. If contact occurs, flush thoroughly with water. Skin irritation may occur, characterized by redness or tingling; if increased, discontinue use and seek medical advice.
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- Questions or Comments?
- Package Labeling:
- 125mL Package Labeling
-
INGREDIENTS AND APPEARANCE
DR. C. TUNA ACNE PORE PURIFYING TONER
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74690-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) DEHYDROACETIC ACID (UNII: 2KAG279R6R) BENZYL ALCOHOL (UNII: LKG8494WBH) GLYCOLIC ACID (UNII: 0WT12SX38S) PHENOXYETHANOL (UNII: HIE492ZZ3T) SALIX ALBA BARK VOLATILE OIL (UNII: PW3MX00JXN) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74690-009-01 1 in 1 BOX 01/20/2021 1 225 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:74690-009-02 1 in 1 BOX 07/25/2021 2 125 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/20/2021 Labeler - Farmasi US LLC (113303351)