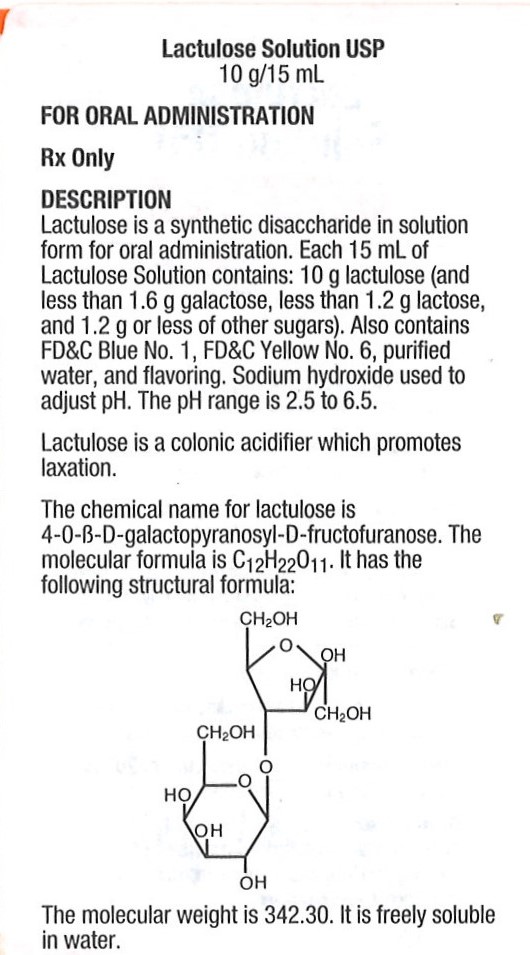
LACTULOSE SOLUTION- lactulose solution solution
TriRx Huntsville Pharmaceutical Services, LLC
----------
Lactulose Solution USP
| LACTULOSE SOLUTION
lactulose solution solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - TriRx Huntsville Pharmaceutical Services, LLC (117090286) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TriRx Huntsville Pharmaceutical Services, LLC | 117090286 | manufacture(80432-001) | |
Revised: 1/2023
Document Id: f1f0ab04-0097-9db4-e053-2995a90aa04c
Set id: b8b710c0-d0ee-3d21-e053-2a95a90ae88f
Version: 2
Effective Time: 20230110
TriRx Huntsville Pharmaceutical Services, LLC