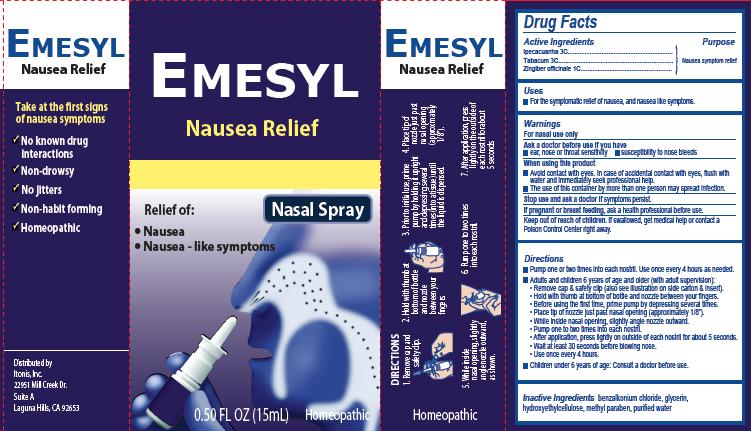
Label: EMESYL NAUSEA RELIEF- ipecacuanha, tabacum, zingiber officinale spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 59067-001-01 - Packager: Itonis, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 31, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor
- If pregnant or breast feeding,
- Keep out of reach of children.
-
Directions
- •
- Pump one or two times into each nostril. Use once every 4 hours as needed.
- •
- Adults and children 6 years of age and older (with adult supervision):
- o
- Remove cap & safety clip (also see illustration on side carton & insert).
- o
- Hold with thumb at bottom of bottle and nozzle between your fingers.
- o
- Before using the first time, prime pump by depressing several times.
- o
- Place tip of nozzle just past nasal opening (approximately 1/8”).
- o
- While inside nasal opening, slightly angle nozzle outward.
- o
- Pump one to two times into each nostril.
- o
- After application, press lightly on outside of each nostril for about 5 seconds.
- o
- Wait at least 30 seconds before blowing nose.
- o
- Use once every 4 hours
- •
- Children under 6 years of age: Consult a doctor before use.
- Inactive Ingredients
-
Principal Display Panel
Emesyl
Nausea Relief
Nasal Spray
Relief of:
•
Nausea
•
Nausea – like symptoms
0.50 FL OZ (15mL) Homeopathic
Take at the first signs of nausea symptomsNo known drug interactions
Non-drowsyNo jitters
Non-habit forming
Homeopathic
Distributed by:
Itonis, Inc.
22951 Mill Creek Dr.
Suite A
Laguna Hills, CA 92653Product Package
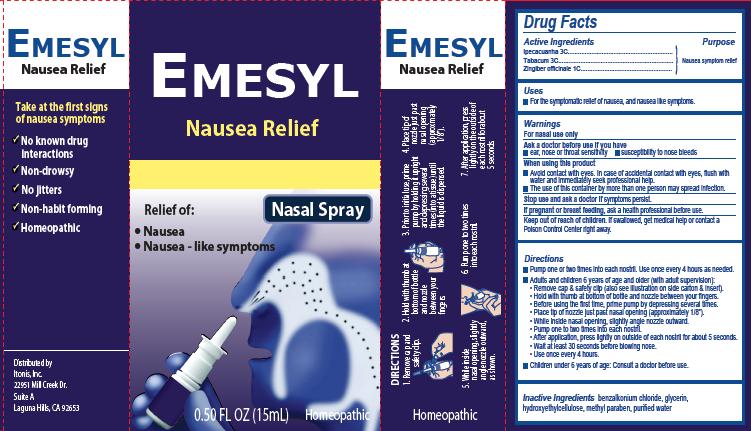
-
INGREDIENTS AND APPEARANCE
EMESYL NAUSEA RELIEF
ipecacuanha, tabacum, zingiber officinale sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59067-001 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 3 [hp_C] in 15 mL TOBACCO LEAF (UNII: 6YR2608RSU) (TOBACCO LEAF - UNII:6YR2608RSU) TOBACCO LEAF 3 [hp_C] in 15 mL GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 1 [hp_C] in 15 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59067-001-01 1 in 1 CARTON 05/20/2014 1 15 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 05/20/2014 Labeler - Itonis, Inc. (829456545)