MENADIONE- menadione, liquid
Apotheca Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Menadione
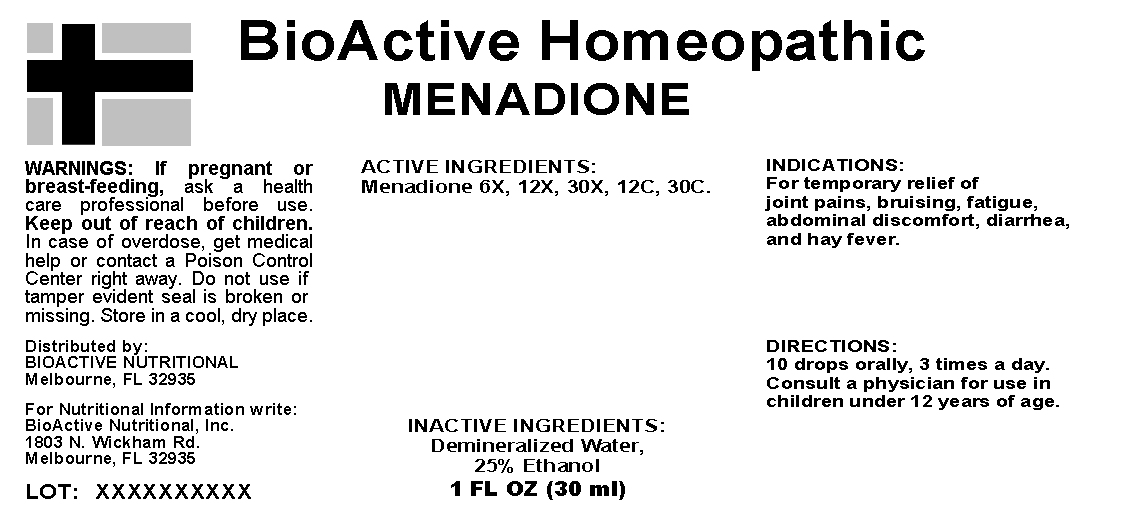
INDICATIONS: For temporary relief of joint pains, bruising, fatigue, abdominal discomfort, diarrhea, and hay fever.
WARNINGS: If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.
INDICATIONS: For temporary relief of joint pains, bruising, fatigue, abdominal discomfort, diarrhea, and hay fever.
| MENADIONE
menadione, liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Apotheca Company (844330915) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(57520-0515) , api manufacture(57520-0515) , label(57520-0515) , pack(57520-0515) | |