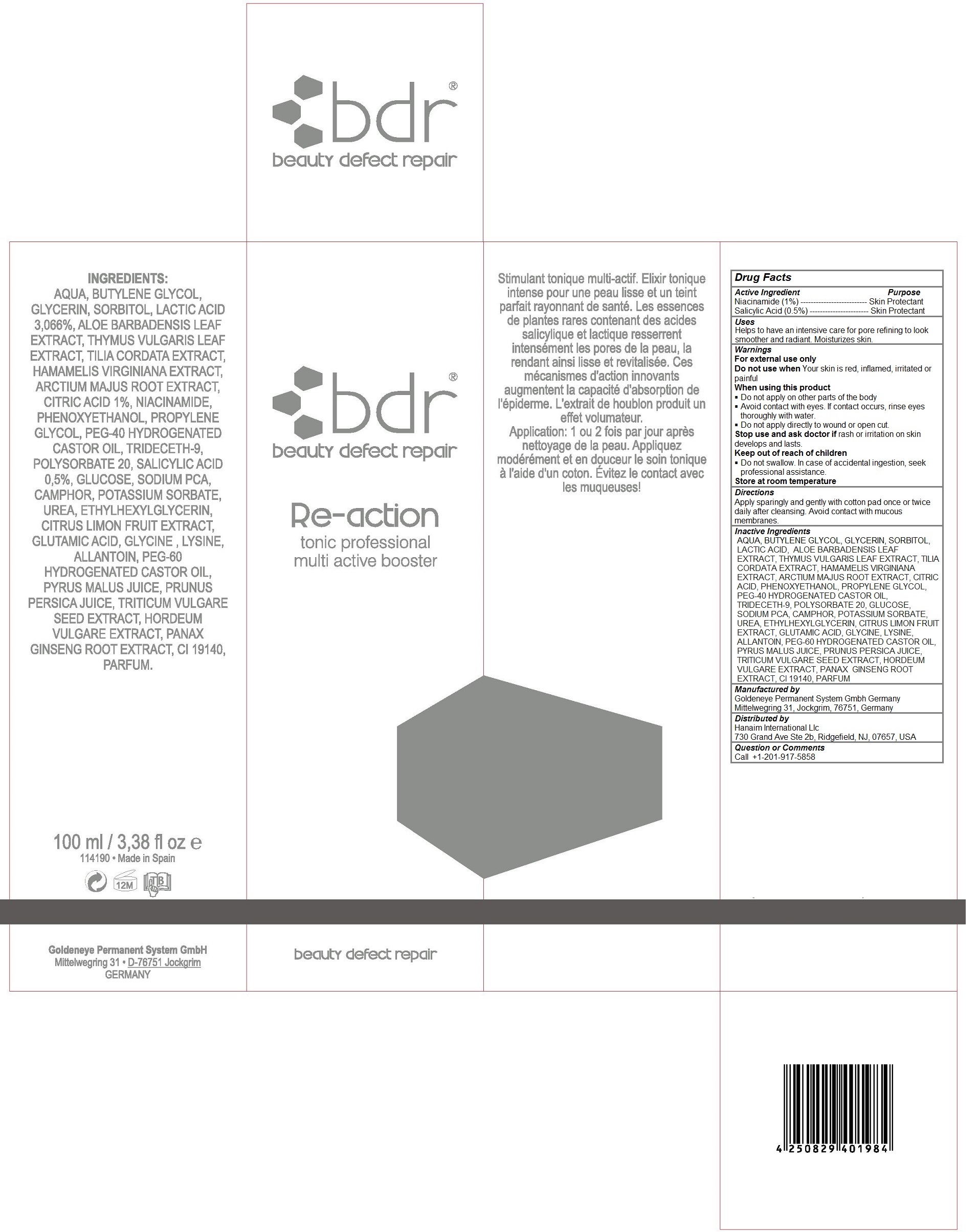
BDR RE-ACTION TONIC PROFESSIONAL MULTI ACTIVE BOOSTER- niacinamide, salicylic acid liquid
Goldeneye Permanent System Gmbh Germany
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
GOLDENEYE - bdr Re-action tonic professional multi active booster
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
Warnings
For external use only
Do not use when Your skin is red, inflamed, irritated or painful
When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on skin develops and lasts.
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
Store at room temperature
Directions
Apply sparingly and gently with cotton pad once or twice daily after cleansing. Avoid contact with mucous membranes
Inactive Ingredients
AQUA, BUTYLENE GLYCOL, GLYCERIN, SORBITOL, LACTIC ACID, ALOE BARBADENSIS LEAF EXTRACT, THYMUS VULGARIS LEAF EXTRACT, TILIA CORDATA EXTRACT, HAMAMELIS VIRGINIANA EXTRACT, ARCTIUM MAJUS ROOT EXTRACT, CITRIC ACID, PHENOXYETHANOL, PROPYLENE GLYCOL, PEG-40 HYDROGENATED CASTOR OIL, TRIDECETH-9, POLYSORBATE 20, GLUCOSE, SODIUM PCA, CAMPHOR, POTASSIUM SORBATE, UREA, ETHYLHEXYLGLYCERIN, CITRUS LIMON FRUIT EXTRACT, GLUTAMIC ACID, GLYCINE, LYSINE, ALLANTOIN, PEG-60 HYDROGENATED CASTOR OIL, PYRUS MALUS JUICE, PRUNUS PERSICA JUICE, TRITICUM VULGARE SEED EXTRACT, HORDEUM VULGARE EXTRACT, PANAX GINSENG ROOT EXTRACT, Cl 19140, PARFUM
| BDR RE-ACTION TONIC PROFESSIONAL MULTI ACTIVE BOOSTER
niacinamide, salicylic acid liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Goldeneye Permanent System Gmbh Germany (329178144) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Goldeneye Permanent System Gmbh Germany | 329178144 | manufacture(71056-101) | |