BEUTHANASIA-D SPECIAL- pentobarbital sodium and phenytoin sodium injection, solution
Merck Sharp & Dohme Corp.
----------
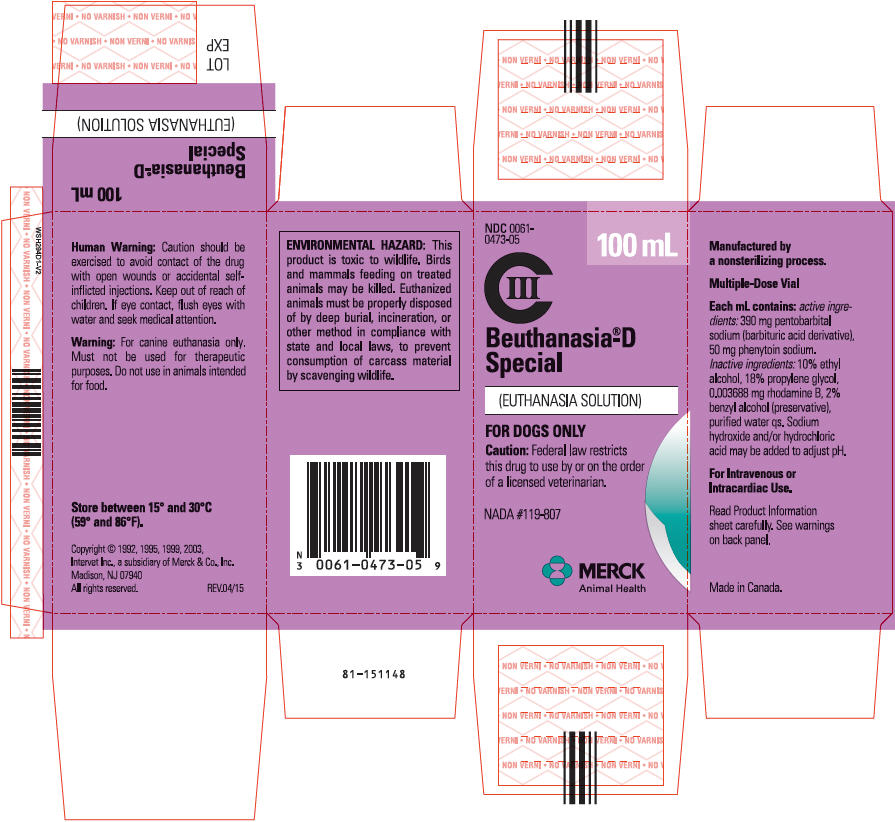
BEUTHANASIA®-D
SPECIAL
(EUTHANASIA SOLUTION)
CIII
FOR DOGS ONLY
NADA #119-807
PRODUCT
INFORMATION
CAUTION Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION A nonsterile solution containing pentobarbital sodium and phenytoin sodium as the active ingredients. Rhodamine B, a bluish-red fluorescent dye, is included in the formulation to help distinguish it from parenteral drugs intended for therapeutic use. Although the solution is not sterile, benzyl alcohol, a bacteriostat, is included to retard the growth of microorganisms.
Each mL contains: active ingredients: 390 mg pentobarbital sodium (barbituric acid derivative), 50 mg phenytoin sodium; inactive ingredients: 10% ethyl alcohol, 18% propylene glycol, 0.003688 mg rhodamine B, 2% benzyl alcohol (preservative), purified water qs. Sodium hydroxide and/or hydrochloric acid may be added to adjust pH.
ACTIONS BEUTHANASIA-D SPECIAL EUTHANASIA SOLUTION contains two active ingredients which are chemically compatible but pharmacologically different. Each ingredient acts in such a manner so as to cause humane, painless, and rapid euthanasia. Euthanasia is due to cerebral death in conjunction with respiratory arrest and circulatory collapse. Cerebral death occurs prior to cessation of cardiac activity.
When administered intravenously, pentobarbital sodium produces rapid anesthetic action. There is a smooth and rapid onset of unconsciousness. At the lethal dose, there is depression of vital medullary respiratory and vasomotor centers.
When administered intravenously, phenytoin sodium produces toxic signs of cardiovascular collapse and/or central nervous system depression. Hypotension occurs when the drug is administered rapidly.
Pharmacodynamic Activity: The sequence of events leading to humane, painless, and rapid euthanasia following intravenous injection of BEUTHANASIA-D SPECIAL EUTHANASIA SOLUTION is similar to that following intravenous injection of pentobarbital sodium or other barbituric acid derivatives. Within seconds, unconsciousness is induced with simultaneous collapse of the dog. This stage rapidly progresses to deep anesthesia with concomitant reduction in the blood pressure. A few seconds later, breathing stops, due to depression of the medullary respiratory center; encephalographic activity becomes isoelectric, indicating cerebral death, and then cardiac activity ceases.
Phenytoin sodium exerts its effect during the deep anesthesia stage caused by the pentobarbital sodium. This ingredient, due to its cardiotoxic properties, hastens the stoppage of electrical activity in the heart.
WARNING For canine euthanasia only. Must not be used for therapeutic purposes. Do not use in animals intended for food.
| ENVIRONMENTAL HAZARD: This product is toxic to wildlife. Birds and mammals feeding on treated animals may be killed. Euthanized animals must be properly disposed of by deep burial, incineration, or other method in compliance with state and local laws, to prevent consumption of carcass material by scavenging wildlife. |
PRECAUTIONS Euthanasia may sometimes be delayed in dogs with severe cardiac or circulatory deficiencies. This may be explained by the impaired movement of the drug to its site of action. An occasional dog may elicit reflex responses manifested by motor movement; however, an unconscious animal does not experience pain, because the cerebral cortex is not functioning.
When restraint may cause the dog pain, injury, or anxiety, or danger to the person making the injection, prior use of tranquilizing or immobilizing drugs may be necessary.
DOSAGE AND ADMINISTRATION
Administration: Intravenous injection is preferred. Intracardiac injection may be made when intravenous injection is impractical, as in a very small dog or in a comatose dog with impaired vascular functions. Good injection skill is necessary for intracardiac injection.
The calculated dose should be given in a single bolus injection.
For intravenous injection, a needle of sufficient gauge to ensure intravenous placement of the entire dose should be used. The use of a Luer-Lok® syringe is recommended to prevent accidental exposure due to needle/syringe separation.
HOW SUPPLIED BEUTHANASIA-D SPECIAL EUTHANASIA SOLUTION is available in 100-mL multiple-dose vials, NDC 0061-0473-05.
Manufactured by a nonsterilizing process.
| BEUTHANASIA-D SPECIAL
pentobarbital sodium and phenytoin sodium injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme Corp. (001317601) |