1ST MEDXPATCH
WITH LIDOCAINE 4%-RX- lidocaine, capsaicin, menthol, methyl salicylate patch
1ST MEDX LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
1ST MEDX (as PLD) - PATCH WITH LIDOCAINE 4%-RX (72137-113) - DELIST
USES:
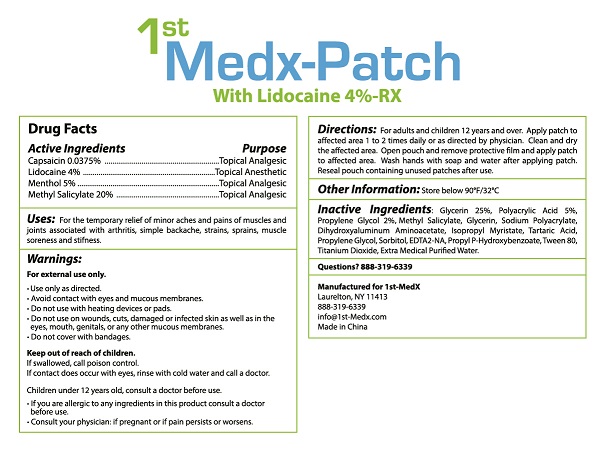
FOR THE TEMPORARY RELIEF OF MINOR ACHES AND PAINS OF MUSCLES AND JOINTS ASSOCIATED WITH ARTHRITIS, SIMPLE BACKACHE, STRAINS, SPRAINS, MUSCLE SORENESS AND STIFFNESS.
WARNINGS:
FOR EXTERNAL USE ONLY.
- USE ONLY AS DIRECTED.
- AVOID CONTACT WITH EYES AND MUCOUS MEMBRANES.
- DO NOT USE ON WOUNDS, CUTS, DAMAGED OR INFECTED SKIN AS WELL AS IN THE EYES, MOUTH, GENITALS, OR ANY OTHER MUCOUS MEMBRANES.
- DO NOT COVER WITH BANDAGES.
IF CONTACT DOES OCCUE WITH EYES, RINSE WITH COLD WATER AND CALL A DOCTOR.
CHILDREN UNDER 12 YEARS OLD, CONSULT A DOCTOR BEFORE USE.
- IF YOU ARE ALLERGIC TO ANY INGREDIENTS IN THIS PRODUCT CONSULT A DOCTOR BEFORE USE.
- CONSULT YOUR PHYSICIAN: IF PREGNANT OR IF PAIN PERSISTS OR WORSENS.
DIRECTIONS:
FOR ADULTS AND CHILDREN 12 YEARS AND OVER. APPLY PATCH TO AFFECTED AREA 1 TO 2 TIMES DAILY OR AS DIRECTED BY PHYSICIAN. CLEAN AND DRY THE AFFECTED AREA. WASH HANDS WITH SOAP AND WATER AFTER APPLYING PATCH. RESEAL POUCH CONTAINING UNUSED PATCHES AFTER USE.
INACTIVE INGREDIENTS:
GLYCERIN 25%, POLYACRYLIC ACID 5%, PROPYLENE GLYCOL 2%, METHYL SALICYLATE, GLYCERIN, SODIUM POLYACRYLATE, DIHYDROXYALUMINUM AMINOACETATE, ISOPROPYL MYRISTATE, TARTARIC ACID, PROPYLENE GLYCOL, SORBITOL, EDTA2-NA, PROPYL P-HYDROXYBENZOATE, TWEEN 80, TITANIUM DIOXIDE, EXTRA MEDICAL PURIFIED WATER.
| 1ST MEDXPATCH
WITH LIDOCAINE 4%-RX
lidocaine, capsaicin, menthol, methyl salicylate patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - 1ST MEDX LLC (081071002) |