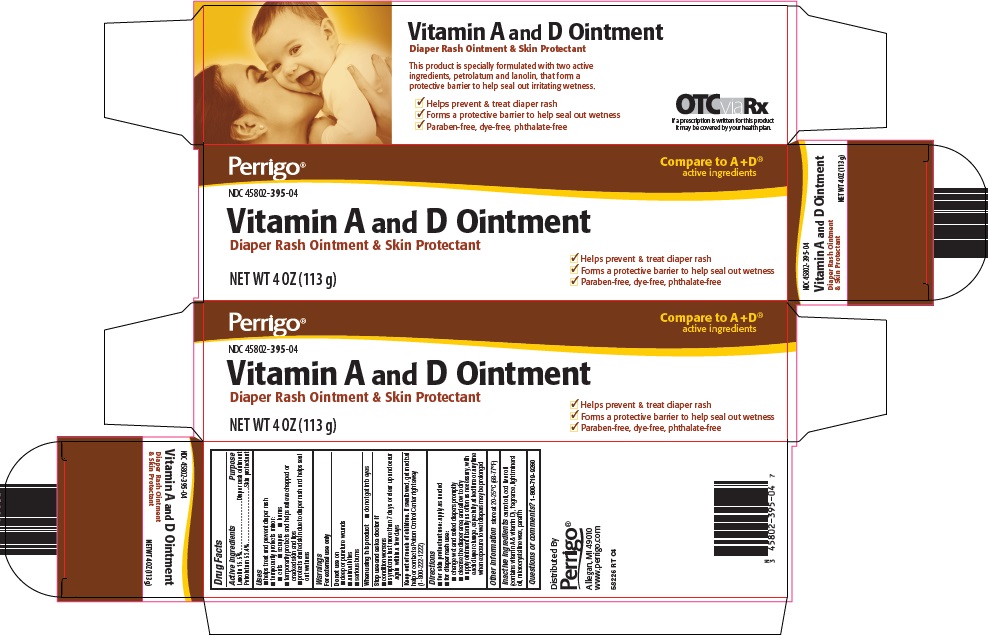
VITAMIN A AND D- lanolin, petrolatum ointment
Padagis Israel Pharmaceuticals Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Perrigo Vitamin A and D Ointment Drug Facts
Uses
- •
- helps treat and prevent diaper rash
- •
- temporarily protects minor:
- •
- cuts
- •
- scrapes
- •
- burns
- •
- temporarily protects and helps relieve chapped or cracked skin and lips
- •
- protects chafed skin due to diaper rash and helps seal out wetness
Warnings
For external use only
Directions
- •
- for skin protectant use: apply as needed
- •
- for diaper rash use:
- •
- change wet and soiled diapers promptly
- •
- cleanse the diaper area, and allow to dry
- •
- apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
| VITAMIN A AND D
lanolin, petrolatum ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Padagis Israel Pharmaceuticals Ltd (600093611) |
Revised: 9/2022
Document Id: adf8eb4a-454d-427d-b2b5-b2949efb0c0b
Set id: b52bab1f-08a9-4aea-be8d-86703e9f0936
Version: 6
Effective Time: 20220912
Padagis Israel Pharmaceuticals Ltd