COLLOIDAL OATMEAL- colloidal oatmeal cream
Vi-Jon
----------
Mountain Falls_Simply U 965.000 965AB
Eczema Soothing Moisturizing Cream
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again withing a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
water, glycerin, distearyldimonium chloride, petrolatum, isopropyl palmitate, cetyl alcohol, panthenol, dimethicone, Avena sativa (oat) kernel oil, steareth-20, Avena sativa (oat) kernel extract, benzalkonium chloride, ceramide NP, sodium chloride, benzyl alcohol, sodium hydroxide
disclaimer
*This product is not manufactured or distributed by Johnson & Johnson Consumer Products Company, distributor of Aveeno® Eczema Therapy Moisturizing Cream.
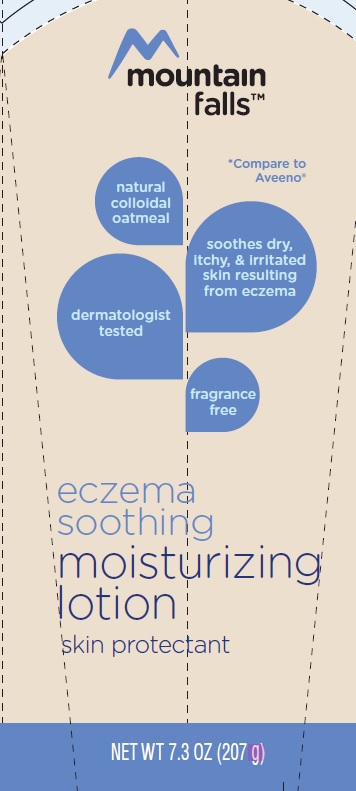
principal panel display
mountain falls
*Compare to Aveeno
natural
colloidal
oatmeal
soothes dry, itchy, & irritated skin resulting from eczema
dermatologist tested
fragrace
free
eczema
soothing
moisturizing
lotion
skin protectant
NET WT 7.3 OZ (207 g)
| COLLOIDAL OATMEAL
colloidal oatmeal cream |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Vi-Jon (088520668) |
| Registrant - Vi-Jon, LLC - Page (088520668) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vi-Jon | 790752542 | manufacture(0869-0965) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vi-Jon | 088520668 | manufacture(0869-0965) | |

