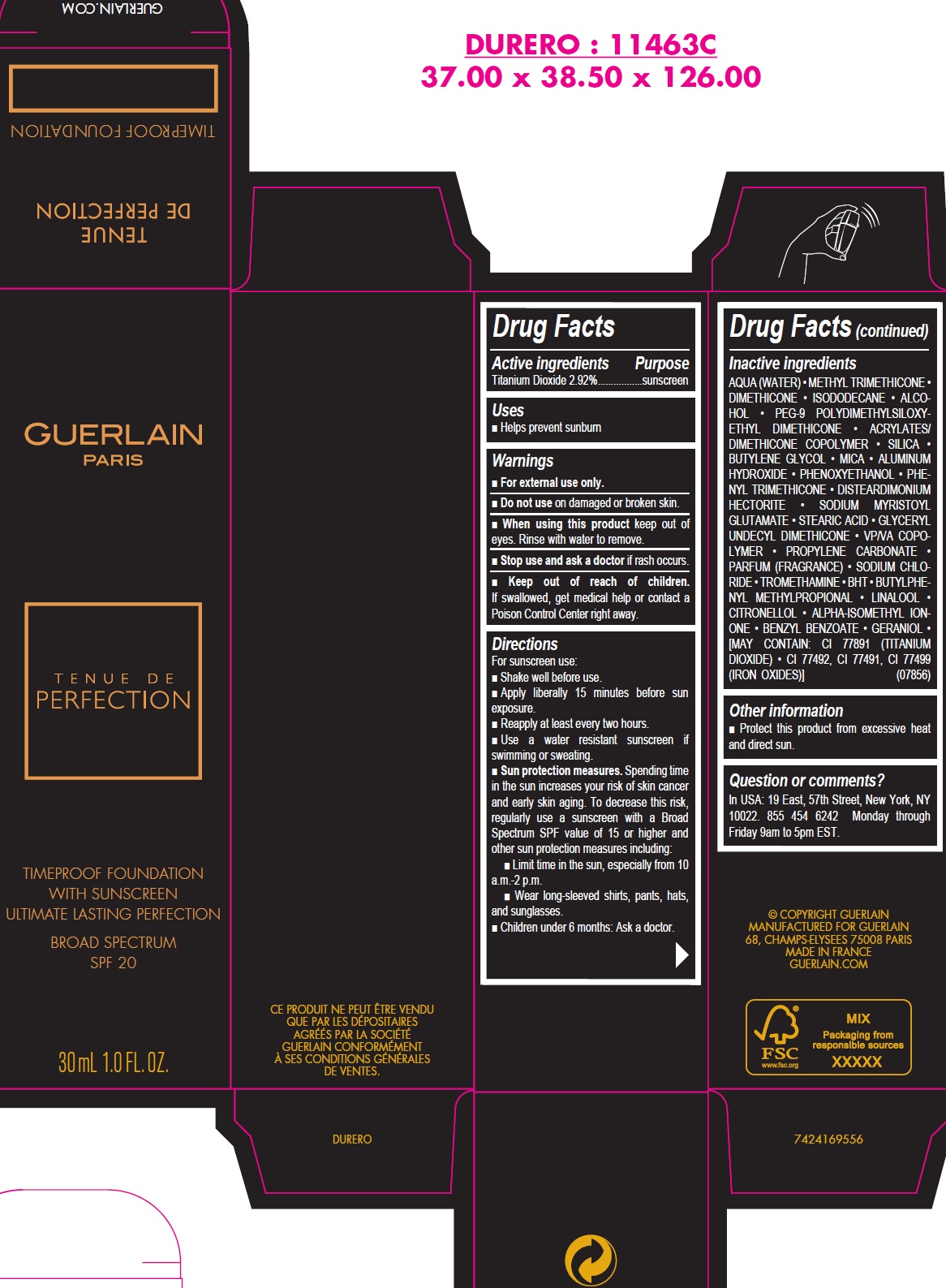
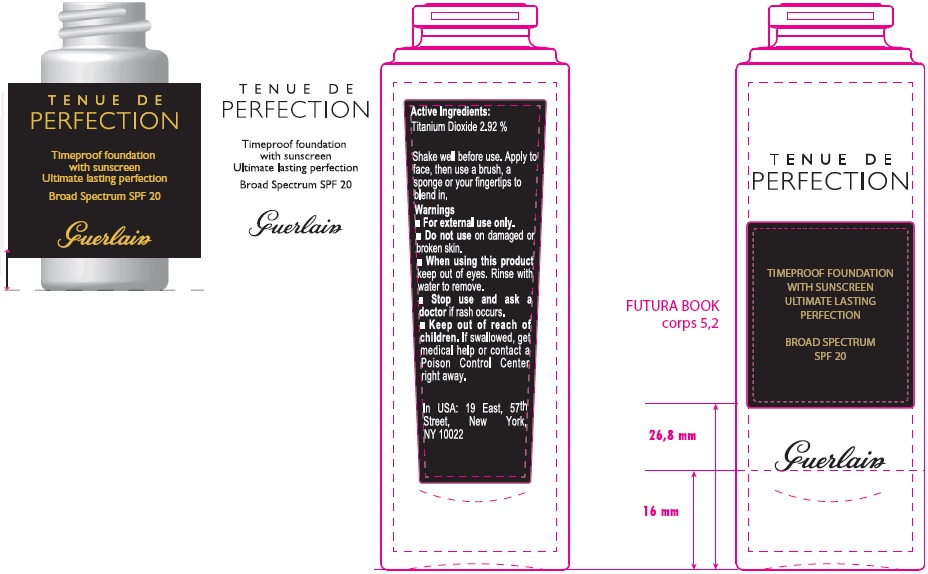
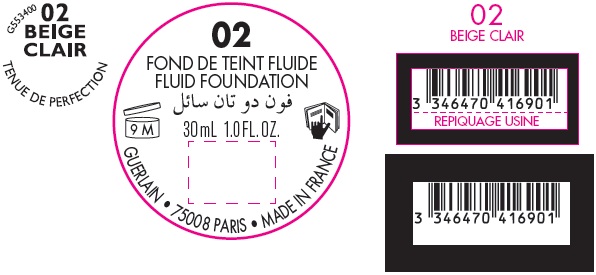
TENUE DE PERFECTION TIMEPROOF FOUNDATION WITH SUNSCREEN ULTIMATE LASTING PERFECTION BROAD SPECTRUM SPF 20 02 BEIGE CLAIR- titanium dioxide emulsion
Guerlain
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
TENUE DE PERFECTION TIMEPROOF FOUNDATION WITH SUNSCREEN ULTIMATE LASTING PERFECTION BROAD SPECTRUM SPF 20 02 BEIGE CLAIR
Directions
For sunscreen use:
- Shake well before use.
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every two hours.
- Use a water resistant sunscreen if swimming or sweating.
- Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor.
Inactive ingredients
AQUA (WATER) • METHYL TRIMETHICONE • DIMETHICONE • ISODODECANE • ALCOHOL • PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE • ACRYLATES/ DIMETHICONE COPOLYMER • SILICA • BUTYLENE GLYCOL • MICA • ALUMINUM HYDROXIDE • PHENOXYETHANOL • PHENYL TRIMETHICONE • DISTEARDIMONIUM HECTORITE • SODIUM MYRISTOYL GLUTAMATE • STEARIC ACID • GLYCERYL UNDECYL DIMETHICONE • VP/VA COPOLYMER • PROPYLENE CARBONATE • PARFUM (FRAGRANCE) • SODIUM CHLORIDE • TROMETHAMINE • BHT • BUTYLPHENYL METHYLPROPIONAL • LINALOOL • CITRONELLOL • ALPHA-ISOMETHYL IONONE • BENZYL BENZOATE • GERANIOL • [MAY CONTAIN: CI 77891 (TITANIUM DIOXIDE) • CI 77492, CI 77491, CI 77499 (IRON OXIDES)] (07856)
| TENUE DE PERFECTION TIMEPROOF FOUNDATION WITH SUNSCREEN ULTIMATE LASTING PERFECTION BROAD SPECTRUM SPF 20 02 BEIGE CLAIR
titanium dioxide emulsion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Guerlain (266623064) |