Label: TISSEEL FIBRIN SEALANT- fibrinogen human, human thrombin kit
TISSEEL FIBRIN SEALANT- fibrinogen human, human thrombin solution
-
NDC Code(s):
0338-4210-02,
0338-4211-04,
0338-4212-10,
0338-4301-02, view more0338-4302-04, 0338-4303-10, 0338-7112-01, 0338-7112-02, 0338-7112-05, 0338-7201-01, 0338-7201-02, 0338-7201-05, 0338-7332-01, 0338-7332-02, 0338-7332-05, 0338-7401-01, 0338-7401-02, 0338-7401-05, 0338-8402-01, 0338-8402-02, 0338-8402-03, 0338-8402-04, 0338-8402-09, 0338-8402-10, 0338-9560-01, 0338-9564-01, 0338-9568-01
- Packager: Baxter Healthcare Corporation
- Category: PLASMA DERIVATIVE
Drug Label Information
Updated December 26, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TISSEEL safely and effectively. See full prescribing information for TISSEEL.
TISSEEL [Fibrin Sealant] For Topical Use Only
Frozen solution and lyophilized powder for solution for topical use only
Initial U.S. Approval: 1998INDICATIONS AND USAGE
Hemostasis: TISSEEL is a fibrin sealant indicated for use as an adjunct to hemostasis in adult and pediatric patients (>1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. TISSEEL is effective in heparinized patients. (1)
Sealing: TISSEEL is a fibrin sealant indicated as an adjunct to standard surgical techniques (such as suture and ligature) to prevent leakage from colonic anastomoses following the reversal of temporary colostomies. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
TISSEEL Kit (Freeze-Dried) is supplied as 2 mL, 4 mL and 10 mL (total volume) pack sizes with and without the DUPLOJECT Fibrin Sealant Preparation and Application System. (3)
TISSEEL Pre-filled Syringe (Frozen) is supplied as 2 mL, 4 mL and 10 mL (total volume) pack sizes with DUO Set (AST syringe) or DUPLOJECT COMBI (PRIMA syringe). (3)
CONTRAINDICATIONS
- •
- Do not inject directly into the circulatory system or into highly vascularized tissue. (4.1, 5.3)
- •
- Do not use in individuals with a known hypersensitivity to aprotinin. (4.2, 5.1, 6)
- •
- Do not use for the treatment of severe or brisk arterial or venous bleeding. (4.3)
- •
- Do not spray where the minimum recommended distance from the applicator tip to the target site cannot be assured. (4.4).
WARNINGS AND PRECAUTIONS
- •
- TISSEEL contains aprotinin, a protein known to be associated with anaphylactic reactions. (4.2, 5.1, 6)
- •
- To reduce the risk of potential life-threatening gas embolism, spray using only the appropriate pressurized gas at the recommended pressure and distance. For Open Surgical procedures, use the EASYSPRAY device connected to CO2, Medical Air or Nitrogen. For Minimally Invasive Surgery procedures use the DUPLOSPRAY MIS device connected only to CO2.(5.2)
- •
- TISSEEL is denatured when exposed to solutions containing alcohol, iodine or heavy metals. (5.2)
- •
- Apply only as a thin layer as excess clot thickness can negatively interfere with wound healing. (2, 5.2)
- •
- When using TISSEEL in surgery do not inject intravascularly. (4.1, 5.3, 6.2).
- •
- Safety has not been evaluated in neurosurgical procedures. (5.4)
- •
- TISSEEL is made from pooled human plasma which can contain infectious agents. (5.5)
ADVERSE REACTIONS
Hypersensitivity or allergic/anaphylactoid reactions have occurred. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare Corporation at 1-888-229-0001 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Oxidized cellulose-containing preparations can reduce the efficacy of TISSEEL and should not be used as carrier materials. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation of TISSEEL Kit (Freeze-Dried)
2.2 Preparation of TISSEEL Pre-Filled Syringe (Frozen)
2.3 Method of Application
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Intravascular Application
4.2 Aprotinin Hypersensitivity
4.3 Severe or Brisk Bleeding
4.4 Application below minimum recommended distance from target site
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Application Precautions
5.3 Use in Surgery
5.4 Use in Neurosurgical Procedures
5.5 Infection Risk from Human Plasma
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Vascular Surgery
14.2 Cardiac Surgery
14.3 Cardiac Reoperations
14.4 Splenectomy
14.5 Colostomy Closure
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Hemostasis: TISSEEL is a fibrin sealant indicated for use as an adjunct to hemostasis in adult and pediatric patients (>1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. TISSEEL is effective in heparinized patients.
Sealing: TISSEEL is a fibrin sealant indicated as an adjunct to standard surgical techniques (such as suture and ligature) to prevent leakage from colonic anastomoses following the reversal of temporary colostomies.
-
2 DOSAGE AND ADMINISTRATION
FOR TOPICAL USE ONLY – DO NOT INJECT
Vials and pre-filled syringes are for single-patient use only. Discard any unused product.
2.1 Preparation of TISSEEL Kit (Freeze-Dried)
Do not expose to temperature above 37ºC
Do not microwave
Do not refrigerate or freeze after reconstitutionDo not use iodine or heavy metal containing preparations such as betadine for disinfection of vial stoppers. Allow alcohol-based disinfectants to evaporate before puncturing stopper.
Use separate syringes and transfer devices for reconstituting Sealer Protein and Thrombin solutions and for application to prevent clotting.
The product must be used within 4 hours after reconstitution.
Freeze-dried Sealer Protein Concentrate and Thrombin are reconstituted in Fibrinolysis Inhibitor Solution and Calcium Chloride Solution, respectively. The Sealer Protein Solution and Thrombin Solution are then combined using the DUPLOJECT Preparation and Application System, or an equivalent delivery device cleared by FDA for use with TISSEEL, to form the Fibrin Sealant.
Pre-warming TISSEEL Kit with FIBRINOTHERM device
See FIBRINOTHERM device manual for complete operating instructions. If a FIBRINOTHERM device is not available, contact Baxter (1-800-229-0001) for assistance.- 1.
- Place all four vials from the TISSEEL Kit into the pre-warmed wells of the FIBRINOTHERM device, using the appropriately sized adapter ring(s), and allow the vials to warm for up to 5 minutes (room temperature product will take less time).
Preparation of Sealer Protein Solution with FIBRINOTHERM device
- 1.
- Remove the caps from the Sealer Protein Concentrate and the Fibrinolysis Inhibitor Solution vials.
- 2.
- Transfer the Aprotinin (Fibrinolysis Inhibitor Solution) into the vial containing the freeze-dried Sealer Protein Concentrate using the sterile reconstitution components (see directions provided with the device system for specific reconstitution instructions). Gently swirl the vial to ensure that the product is completely soaked.
- 3.
- Place the vial into the largest opening of the FIBRINOTHERM device with the appropriate adapter ring. Switch on the stirrer and allow the vial contents to stir until all Sealer Protein Concentrate is dissolved. Reconstitution is complete when no undissolved particles are visible.
Notes:
- -
- If the Sealer Protein Concentrate has not fully dissolved within 20 minutes discard the vial and prepare a fresh kit.
- -
- Keep the Sealer Protein Solution at 37°C without stirring. Stir shortly before drawing up the solution to ensure homogeneity.
Preparation of Thrombin Solution with FIBRINOTHERM device
To reconstitute the Thrombin (Human) with the Calcium Chloride Solution; follow steps 1-3 under Preparation of Sealer Protein with FIBRINOTHERM deviceutilizing the Thrombin and Calcium Chloride vials.
Transferring to the Sterile Field
For transfer of the Sealer Protein and Thrombin Solutions to the sterile field, the circulating nurse should disinfect the tops of the vials with a germicidal solution and allow to dry. The scrub nurse should withdraw the sterile solutions while the circulating nurse holds the non-sterile vials. Slowly withdraw the solution, by firm constant aspiration, to reduce the risk of large air bubbles.
2.2 Preparation of TISSEEL Pre-Filled Syringe (Frozen)
Do not expose to temperature above 37˚C Do not microwave.
Do not refrigerate or re-freeze after thawing.
Do not use TISSEEL (frozen) until it is completely thawed and warmed (liquid consistency) to 33-37oC. Do not remove the protective syringe cap until use.AST Syringe – The plunger rod must be inserted into the syringe barrel (see ).
PRIMA Syringe – The plunger is already assembled with the syringe barrel (see Figure 2).Sterile Water Bath (Quick Thawing): Transfer inner pouch to the sterile field, remove pre-filled syringe from inner pouch and place directly into sterile water bath ensuring the syringe is completely immersed in the water. Maintain the product at 33-37°C until use. Once the package is opened or the product is warmed to 33-37°C, it must be used within 4 hours.
Non-Sterile Water Bath: Maintain the pre-filled syringe in pouches and place into a water bath outside the sterile field ensuring the pouches remain submerged. Remove from the water bath after thawing and warming, dry the external pouch and transfer inner pouch with pre-filled syringe onto the sterile field. Maintain the product at 33-37°C until use. Once the package is opened or the product is warmed to 33-37°C, it must be used within 4 hours.
Incubator: Maintain the pre-filled syringe in pouches and place into an incubator. Remove from the incubator after thawing and warming. Transfer inner pouch with pre-filled syringe onto the sterile field. Maintain the product at 33-37°C until use. Once the package is opened or the product is warmed to 33-37°C, it must be used within 4 hours.
Table 1: Approximate Water Bath or Incubator Thawing and Warming Times Pack Size
Sterile Water Bath
(Pouches Removed)
33 - 37°C
Non-Sterile Water Bath
(In Pouches)
33 - 37°C
Incubator
(In Pouches)
33 - 37°C
PRIMA Syringe
AST Syringe
PRIMA Syringe
AST Syringe
PRIMA Syringe
AST Syringe
2 mL
5 minutes
5 minutes
15 minutes
30 minutes
40 minutes
40 minutes
4 mL
5 minutes
5 minutes
20 minutes
40 minutes
50 minutes
85 minutes
10 mL
10 minutes
12 minutes
35 minutes
80 minutes
90 minutes
105 minutes
Room Temperature Thawing: Unopened pouches can be stored for up to 48 hours at room temperature (15-25°C). Before use, warm the product to 33-37°C and apply immediately. The total thawing and warming time cannot exceed 48 hours.
Table 2: Approximate Room Temperature Thawing Times Pack Size
Room Temperature
(In Pouches)
15 – 25oC
PRIMA Syringe
AST Syringe
2 mL
80 minutes
60 minutes
4 mL
90 minutes
110 minutes
10 mL
160 minutes
160 minutes
Table 3: Approximate Water Bath or Incubator Warming Times for Thawed Product Pack Size
Sterile Water Bath
(Pouches Removed)
33 – 37°C
Non-Sterile Water Bath
(In Pouches)
33 – 37°C
Incubator
(In Pouches)
33 – 37°C
PRIMA Syringe
AST Syringe
PRIMA Syringe
AST Syringe
PRIMA Syringe
AST Syringe
2 mL
2 minutes
2 minutes
5 minutes
16 minutes
16 minutes
20 minutes
4 mL
2 minutes
2 minutes
8 minutes
21 minutes
21 minutes
43 minutes
10 mL
4 minutes
6 minutes
12 minutes
43 minutes
35 minutes
52 minutes
2.3 Method of Application
TISSEEL Kit (Freeze-Dried)
Apply TISSEEL using the DUPLOJECT Fibrin Sealant Preparation and Application System or an equivalent delivery system (including open and minimally invasive spray devices) cleared by FDA for use with TISSEEL. Specific instructions for the use of TISSEEL in conjunction with each cleared delivery device are provided with the device.
TISSEEL Pre-filled Syringe Frozen
Apply pre-filled TISSEEL using the joining piece and application cannula accessory devices provided with the product or an equivalent delivery device (including open and minimally invasive spray devices) cleared by the FDA for use with TISSEEL.
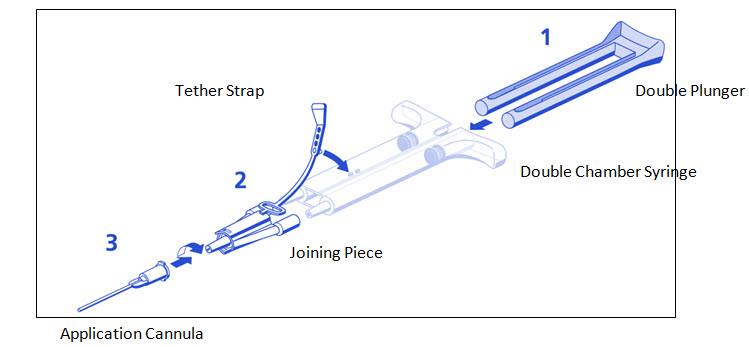
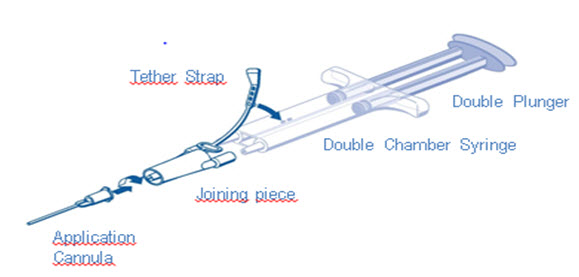
DUO Set (AST Syringe) Instructions (see ):
- 1.
- Insert plunger into syringe barrel.
- 2.
- Remove all air from the double chamber syringe.
- 3.
- Firmly connect the two syringe nozzles to the joining piece (Y-connector) and secure it by fastening the tether strap to the syringe.
- 4.
- Fit an application cannula to the joining piece. Apply by depressing plunger.
DUPLOJECT COMBI (PRIMA Syringe) Instructions (see )
- 1.
- The plunger is attached to the syringe barrel and does not need to be inserted.
- 2.
- Remove all air from the double chamber syringe.
- 3.
- Firmly connect the two syringe nozzles to the joining piece (Y-connector) and secure it by fastening the tether strap to the syringe.
- 4.
- Fit an application cannula to the joining piece. Apply by depressing plunger.
- Note: Interruption of TISSEEL application causes clogging in the cannula. Replace the cannula immediately prior to resuming application. If the opening of the joining piece (Y-connector) facing the cannula is clogged, use the spare joining piece provided in the package.
TISSEEL must be sprayed only onto application sites that are visible. Dry the site of application as much as possible. The surface area of the wound needs to be dried using standard techniques (e.g. intermittent application of compresses, swabs, use of suction devices). Do not use pressurized air or gas to dry the site.
When applying TISSEEL using a spray device, utilize the recommended gas, pressure and distance from tissue within the ranges recommended by the manufacturer as follows:
Table 4: Recommended Application Equipment, Gas and Parameters - *
- Medical grade CO2 is the preferred gas for application, however compressed Air or Nitrogen are acceptable gasses for administration of TISSEEL in open surgery.
Surgery
Spray set / Applicator tips to use
Pressure regulator to use
Gas
Distance
Spray Pressure
Open surgery
TISSEEL /ARTISS
Spray Set
EASY SPRAY
Pressure Regulator
Medical grade CO2*, Compressed
Air or Nitrogen
10-15 cm
1.5-2.0 bar
(21.8-29.0 psi)
Laparoscopic/ minimally invasive procedures
DUPLOSPRAY MIS Applicator 20 cm
DUPLOSPRAY
MIS Regulator
CO2 Only
Range 2-5 cm
3 cm recommended
1.18-1.50 bar
(17-22 psi)
DUPLOSPRAY MIS Applicator 30 cm
DUPLOSPRAY MIS Applicator 40 cm
360º Flexible
Applicator 40 cm
Replaceable tip
Apply TISSEEL as a thin layer by dripping or spraying using a cannula or spray set approved for use with TISSEEL. To reduce the risk of potentially life-threatening gas embolism, spray TISSEEL using only the appropriate pressurized gas within the pressure range and distance recommended in the device Instructions For Use (see Warnings and Precautions (5.2)). The treating physician will determine the amount of TISSEEL to be applied based on the surface to be covered. Ensure that the amount applied is sufficient to entirely cover the intended application area. The approximate surface areas covered by each package size of TISSEEL are listed in Table 5:
Table 5: Surface Area Coverage Required package size of
TISSEEL
Maximum coverage using spray
Maximum coverage using cannula
2 mL
4 mL
10 mL100 cm2
200 cm2
500 cm28 cm2
16 cm2
40 cm2Avoid application beyond the intended area. Allow at least 2 minutes after application to achieve sufficient polymerization. If repeat application is needed, dry the site as much as possible before reapplying. Reapply after removing residues from the prior application or before polymerization takes place since TISSEEL may not adhere firmly to a polymerized layer.
In cases where very small volumes (1-2 drops) are required, expel and discard the first several drops from the application cannula immediately before application to ensure administration of adequately mixed TISSEEL.
-
3 DOSAGE FORMS AND STRENGTHS
TISSEEL Kit (Freeze-Dried) is supplied as 2 mL, 4 mL and 10 mL (total volume) pack sizes with and without the DUPLOJECT Fibrin Sealant Preparation and Application System. TISSEEL Pre-Filled Syringe (Frozen) is supplied as 2 mL, 4 mL and 10 mL (total volume) pack sizes with the DUO Set (AST Syringe) or DUPLOJECT COMBI (PRIMA Syringe).
-
4 CONTRAINDICATIONS
4.1 Intravascular Application
Do not inject TISSEEL directly into the circulatory system or into highly vascularized tissue. Intravascular application of TISSEEL can lead to intravascular coagulation, can result in life-threatening thromboembolic events, and can increase the likelihood and severity of acute hypersensitivity reactions in susceptible patients (see Warnings and Precautions (5.3) and Adverse Reactions (6.2)).
4.2 Aprotinin Hypersensitivity
Do not use TISSEEL in individuals with a known hypersensitivity to aprotinin (see Warnings and Precautions (5.1) and Adverse Reactions (6)).
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions including allergic/and anaphylactoid reactions can occur with the use of TISSEEL. Cases have been reported in post marketing experience with Baxter’s fibrin sealant (see Adverse Reactions (6.2)). In specific cases, these reactions have progressed to severe anaphylaxis. Such reactions may especially be seen if TISSEEL is applied repeatedly over time or in the same setting, or if systemic aprotinin has been administered previously. Even if the first treatment was well tolerated, this may not exclude the occurrence of an allergic reaction after a subsequent administration of TISSEEL or systemic aprotinin. Observed symptoms of allergic anaphylactic reactions to TISSEEL have included: bradycardia, tachycardia, hypotension, flushing, bronchospasm, wheezing, dyspnea, nausea, urticaria, angioedema, pruritus, erythema and paresthesia. Such reactions can also occur in patients receiving TISSEEL for the first time.
Aprotinin is included in TISSEEL for its antifibrinolytic properties. Aprotinin, a protein, is known to be associated with anaphylactic reactions. Even in the case of strict local application of aprotinin, there is a risk of anaphylactic reactions to aprotinin, particularly in the case of previous exposure (see Contraindications (4.2)). TISSEEL does not contain any substances of bovine origin.
Discontinue administration of TISSEEL in the event of hypersensitivity reactions. Mild reactions can be managed with antihistamines. Severe hypotensive reactions require immediate intervention using current principles of shock therapy. Remove remaining product from the application site.
5.2 Application Precautions
Any application of pressurized air or gas is associated with a potential risk of air or gas embolism, tissue rupture, or gas entrapment with compression, which may be life a threatening or fatal.
Life threatening/fatal air or gas embolism has occurred when Fibrin Sealants were administered using pressurized gas with open regulator spray devices. This can occur if a spray device is used at higher than recommended pressures and in closer than recommended proximity to the tissue surface. The solubility of compressed CO2 is greater than either compressed N2 or Air thereby reducing the potential effect of embolization.
Regardless of the type of gas used, to reduce the incidence of embolization, spray TISSEEL using only the recommended regulator, set within the recommended pressure range, with the appropriate applicator positioned at the recommended distance in .
Monitor changes in blood pressure, pulse, oxygen saturation and endtidal CO2 due to the possibility of air or gas embolism.
Use only spray catheters or applicators approved for use with TISSEEL.
TISSEEL must not be sprayed in enclosed body areas using the EASYSPRAY device and must be sprayed onto only visible application sites.
For Open Surgical procedures, use the EASYSPRAY Pressure Regulator connected to medical grade CO2, compressed Air or a Nitrogen compressed gas source along with the TISSEEL/ARTISS spray set, (see Method of Application (2.3)).
For Minimally Invasive Surgery proceduresin enclosed body areas use of the DUPLOSPRAY MIS device connected only to compressed CO2, along with DUPLOSPRAY applicator is recommended. The DUPLOSPRAY MIS device is specifically designed to prevent over pressurization of the body cavity through a dedicated ventline to reduce the risk of gas embolization, (see Method of Application (2.3)).
The sealer protein and thrombin solutions are denatured by alcohol, iodine or heavy metal ions. If any of these substances have been used to clean the wound area, the area must be thoroughly rinsed before the application of TISSEEL.
Apply TISSEEL as a thin layer as excess clot thickness can negatively interfere with wound healing.
5.3 Use in Surgery
When using TISSEEL in surgery, do not inject intravascularly (see Contraindications (4.1) and Adverse Reactions (6.2)).
5.4 Use in Neurosurgical Procedures
The safety and effectiveness of TISSEEL used alone or in combination with biocompatible carriers in neurosurgical procedures or other surgeries involving confined spaces have not been evaluated, and its use in this setting is not approved by FDA (see Adverse Reactions (6.2) and Drug Interactions (7)).
5.5 Infection Risk from Human Plasma
TISSEEL is made from human plasma. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically the Creutzfeldt-Jakob disease (CJD) agent.
All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Baxter Healthcare Corporation at 1-888-229-0001.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Increased D-Dimer levels have been observed during a clinical study in cardiovascular surgery (see Clinical Studies (14)), but did not exceed values reported in the literature occurring after this type of surgery. Postoperatively increased D-Dimers can result at least partly from the degradation of Fibrin Sealant.
There were no reports of serious, associated adverse reactions reported above 1% in clinical studies.
6.2 Post-Marketing Experience
Because adverse reactions are reported voluntarily and the population is of uncertain size, it is not always possible to reliably estimate the frequency of these reactions.
The following adverse reactions have been reported in the post-marketing experience.
Immune System Disorders: Hypersensitivity, including anaphylactic reaction and anaphylactic shock. Anaphylactic reactions and anaphylactic shock have included fatal outcomes.
Vascular Disorders:Hypotension, flushing, embolism, including cerebral artery embolism, cerebral infarction*, air embolism**
Skin and subcutaneous Tissue Disorders:Angioedema, erythema, impaired healing, pruritus, urticaria
Cardiac Disorders:Bradycardia, tachycardia
Respiratory Disorders: Bronchospasm, dyspnea, wheezing
Gastrointestinal Disorders:Nausea
Nervous System Disorders:Paresthesia
* as a result of intravascular application into the superior petrosal sinus
** As with other fibrin sealants life-threatening/fatal air or gas embolism when using devices with pressurized air or gas occurred; this event appears to be related to an inappropriate use of the spray device (e.g. at higher than recommended pressure and in close proximity to the tissue surface),Class effect: Manifestations of hypersensitivity or allergic reactions associated with the class of fibrin sealant/hemostatic products include: application site irritation, chest discomfort, chills, headache, lethargy, restlessness and vomiting.
There have been reports of serious adverse events such as paralysis and other compressive complications possibly related to the use of fibrin sealants in combination with resorbable hemostatic agents. There have also been reports of fatalities following the misadministration of topical thrombin (see Warnings and Precautions (5.4)).
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no direct or controlled studies of TISSEEL in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with TISSEEL. It is also not known whether TISSEEL can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Some viruses, such as parvovirus B19, are particularly difficult to remove or inactivate. Parvovirus B19 most seriously affects pregnant women (fetal infection). In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of TISSEEL in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for TISSEEL and any potential adverse effects on the breastfed infant from TISSEEL or from underlying maternal condition.
8.4 Pediatric Use
Limited clinical study data are available with regard to the use of TISSEEL in children. Of 365 patients undergoing repeated cardiac surgery or emergency resternotomy in a clinical trial of TISSEEL, 27 pediatric patients aged 16 years or younger were treated with TISSEEL. Of these, 2 patients were less than 6 months, 2 patients were between the ages of 6 months and 2 years, 15 patients were between 3-11 years of age, and 8 patients were between 12-16 years of age. There were no differences in safety observed between these subjects and the overall population. (seeClinical Studies (14)).
8.5 Geriatric Use
Clinical studies included 218 patients aged 65 years of age or older treated with TISSEEL (159 undergoing cardiac surgery and 59 undergoing vascular surgery) (see Clinical Studies (14)). No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
TISSEEL [Fibrin Sealant] is a two-component fibrin sealant made from pooled human plasma. When combined, the two components, Sealer Protein and Thrombin mimic the final stage of the blood coagulation cascade.
Sealer Protein (Human)
Sealer Protein (Human) is a sterile, non-pyrogenic, vapor heated and solvent/detergent treated preparation made from pooled human plasma. Sealer Protein (Human) is provided either as a freeze-dried powder for reconstitution with Aprotinin or as a finished frozen solution pre-filled into one side of a dual-chambered syringe. The active ingredient in Sealer Protein (Human) is fibrinogen. Sealer Protein (Human) solution contains fibrinolysis inhibitor, synthetic Aprotinin, that delays fibrinolysis. Aprotinin (Synthetic) is manufactured by solid phase synthesis from materials completely of non-human/non-animal origin.Thrombin (Human)
Thrombin (Human) is a sterile, non-pyrogenic, vapor heated and solvent/detergent treated preparation made from pooled human plasma. Thrombin (Human) is also provided either as a freeze-dried powder for reconstitution with Calcium Chloride Solution or as a finished frozen solution pre-filled into one side of a dual-chambered syringe.- The reconstituted solution or pre-filled syringe contains:
Sealer Protein Solution - Total protein: 96 – 125 mg/mL
Fibrinogen: 67 – 106 mg/mL
Aprotinin (Synthetic): 2250 – 3750 KIU/mL
Other ingredients include: human albumin, tri-sodium citrate, histidine, niacinamide, polysorbate 80 and water for injection.- Thrombin Solution
- Thrombin (Human): 400 – 625 units/mL*
Calcium Chloride: 36 – 44 µmol/mL
Other ingredients include: human albumin, sodium chloride and water for injection. - * The potency expressed in units is determined with a clotting assay using an in-house internal standard that has been calibrated against the World Health Organization (WHO) Second International Standard for Thrombin, 01/580. Therefore, a unit (U) is equivalent to an International Unit (IU).
Viral Clearance
TISSEEL is made from pooled human plasma collected at US licensed collection centers. The vapor heat and solvent/detergent treatment steps used in the manufacturing process have been shown to be capable of significant viral reduction. No procedure, however, has been shown to be completely effective in removing viral infectivity from derivatives of human plasma (see Warnings and Precautions (5.5)). Validation studies were conducted using samples drawn from manufacturing intermediates for each of the two human plasma derived components. These samples were spiked with stock virus suspensions of known titers followed by further processing under conditions representative of respective manufacturing steps.The virus reduction factors (expressed as log10) of manufacturing steps for each of the viruses tested are shown in .
Table 6: Reduction Factors for Virus Removal and/or Inactivation Sealer Protein Component
Manufacturing Step
Mean Reduction Factors [log10] of Virus Tested
HIV-1
HAV
BVDV
PRV
MMV
Early Manufacturing Steps
n.d.
n.d.
n.d.
n.d.
2.7
Solvent/Detergent Treatment
>5.3
n.d.
>5.7
>5.9
n.d.
Vapor Heat Treatment
>5.5
>5.6
>5.7
>6.7
1.2
Overall Reduction Factor (ORF)
>10.8
>5.6
>11.4
>12.6
3.9
Thrombin Component
Manufacturing Step
Mean Reduction Factors [log10] of Virus Tested
HIV-1
HAV
BVDV
PRV
MMV
Thrombin Precursor Mass Capture
3.2
1.5
1.8
2.5
1.2
Vapor Heat Treatment
>5.5
>4.9
>5.3
>6.7
1.0
Solvent/Detergent Treatment
>5.3
n.d.
>5.5
>6.4
n.d.
Ion Exchange Chromatography
n.d.
n.d.
n.d.
n.d.
3.6
Overall Reduction Factor (ORF)
>14.0
>6.4
>12.6
>15.6
5.8
n.d. = not determined
HIV-1: Human Immunodeficiency Virus 1; HAV: Hepatitis A Virus; BVDV: Bovine Viral Diarrhea Virus, a model for Hepatitis C Virus; PRV: Pseudorabies Virus, a model for lipid enveloped DNA viruses, among those is Hepatitis B Virus; MMV: Mouse Minute Virus, a model for B19V.
In addition, Human Parvovirus B19 (B19V) was used to investigate the upstream Thrombin precursor mass capture step, the Sealer Protein early manufacturing steps and the Thrombin and Sealer Protein vapor heating steps. Using quantitative PCR assays, the estimated B19V log reduction factors were: (a) 1.7 for the Thrombin precursor mass capture step, (b) 3.4 for Sealer Protein early manufacturing steps, (c) >4 for Thrombin vapor heat treatment and (d) 1.0 for Sealer Protein vapor heat treatment.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Upon mixing Sealer Protein (Human) and Thrombin (Human), soluble fibrinogen is transformed into fibrin, forming a rubber-like mass that adheres to the wound surface and achieves hemostasis and sealing or gluing of tissues. TISSEEL mimics the final coagulation cascade step as it has all relevant components to form a clot. TISSEEL is effective in heparinized patients and in patients medicated with anti-platelet drugs.
12.2 Pharmacodynamics
Thrombin is a highly specific protease that transforms the fibrinogen contained in Sealer Protein (Human) into fibrin. Fibrinolysis inhibitor, Aprotinin (Synthetic), is a polyvalent protease inhibitor that prevents premature degradation of fibrin. Preclinical studies with different fibrin sealant preparations simulating the fibrinolytic activity generated by extracorporeal circulation in patients during cardiovascular surgery have shown that incorporation of aprotinin in the product formulation increases resistance of the fibrin sealant clot to degradation in a fibrinolytic environment.
12.3 Pharmacokinetics
Unincorporated Aprotinin and its metabolites have a half-life of 30 to 60 minutes and are eliminated by the kidney. Pharmacokinetic studies were not conducted. TISSEEL is expected to be completely resorbed in 10 to14 days
Because TISSEEL is applied only topically, systemic exposure or distribution to other organs or tissues is not expected.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Vascular Surgery
TISSEEL was evaluated in a prospective, controlled, randomized, single-blind, multicenter clinical study against manual compression with gauze pads in 140 subjects undergoing vascular surgery with expanded polytetrafluoroethylene (ePFTE) graft placement (arterio-arterial bypasses and AV shunts for dialysis access in the upper and lower extremity). Subjects received standardized dosages of heparin. Protamine was administered after the primary endpoint had been assessed. Long-term antiplatelet treatments were continued perioperatively at the surgeon’s discretion.
Subjects were randomly assigned to TISSEEL or control when persistent bleeding at the study suture line was present after surgical hemostasis, i.e., sutures. Eligible bleedings before clamping and treatment application were defined as a minimum of 25% of the suture line bleeds or at least 5 suture line bleedings or any pulsatile or spurting needle hole bleeding. For the primary endpoint, hemostasis achieved at the study suture line at 4 minutes and maintained until surgical closure, a single application of TISSEEL was statistically significantly superior to control (p<0.0001; likelihood ratio chi-square test; 2.5% one sided) [ITT].
Table 7: Vascular Surgery Hemostasis at the study suture line within 4 minutes and maintained until surgical closure
TISSEEL
Manual Compression
Intent to Treat Analysis
44/70 (62.9%)
22/70 (31.4%)
14.2 Cardiac Surgery
TISSEEL was evaluated in a prospective, parallel design, randomized (1:1), double-blind, multicenter clinical study against an earlier formulation of the product, TISSEEL VH, in 317 subjects undergoing cardiac surgery requiring cardiopulmonary bypass (CPB) and median sternotomy. Patients were treated with TISSEEL or the control product only when hemostasis was not achieved by conventional surgical methods. For the endpoint, hemostasis achieved at the primary treatment site within 5 minutes of treatment and maintained until closure of the surgical wound, TISSEEL was non-inferior to the earlier formulation of the product using a one-sided 97.5% confidence interval on the difference in the proportion of subjects successfully treated.
Table 8: Cardiac Surgery Hemostasis within 5 minutes and maintained until surgical closure
TISSEEL
TISSEEL VH
Intent to Treat Analysis
127/144 (88.2%)
129/144 (89.6%)
14.3 Cardiac Reoperations
An earlier formulation of TISSEEL was evaluated in an open-label crossover study against control topical hemostatic agents in 489 patients undergoing cardiovascular reoperation or resternotomy at 11 institutions. Patients were randomized to TISSEEL or control hemostatic agents when a topical hemostatic was needed at the conclusion of surgery and after all attempts at surgical hemostasis. Patients were crossed to the alternative therapy if bleeding continued after the 5 minute endpoint. At 10 centers, TISSEEL was used after administration of protamine sulfate. At one site, TISSEEL could be used before administration of protamine sulfate. 365 of the 489 patients developed bleeding episodes requiring treatment. For the endpoint (successful hemostasis at 5 minutes), TISSEEL was statistically significantly superior to control topical hemostatic agents in these patients. Similarly, absolute time to cessation of bleeding was statistically significantly shorter for TISSEEL than for control topical hemostatic agents (p<0.0001, Gehan- Wilcoxon test, two sided).
Table 9: Cardiac Reoperations Hemostasis within 5 minutes
TISSEEL
Control Topical Hemostatic Agent
82.4% (159/193)
44.5% (76/172)
Pearson χ2 two sided; p <0.0001; intent-to-treat analysis
14.4 Splenectomy
In a single center, open label trial, an earlier formulation of TISSEEL was compared to historical controls in patients undergoing laparotomy for blunt or penetrating traumatic injury to the spleen and/or liver. Use of TISSEEL resulted in the need for statistically significantly fewer splenectomies than control hemostatic maneuvers (Refer to Table 9). TISSEEL did not result in significantly reduced mortality in patients with blunt or penetrating trauma to the liver alone or to the liver and spleen (p=0.067, χ2, one sided).
Table 10: Splenectomy Splenectomy Rate
Injury to:
TISSEEL
Historic Controls
Spleen
0/19
14/22
p <0.001
Spleen and liver
1/26
19/34
p <0.001
14.5 Colostomy Closure
In a single center, prospective open label study of 118 patients randomized to standard of care (58 patients) or standard of care plus fibrin sealant (60 patients) for elective colostomy closure after temporary colostomy placement for treatment of traumatic injury to the colon, the earlier version of TISSEEL plus standard of care was also shown to be significantly superior to standard of care alone (p=0.0406, Jonckheere-Terpstra test for ordinal data, two sided) with regard to anastomotic complications (leakage, intra-abdominal abscess formation, re-operation, septic shock, and death).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
TISSEEL is supplied in the following pack sizes and presentations:
Table 11: NDC Numbers Pack Size
- TISSEEL Kit (Freeze-Dried)
TISSEEL Kit (Freeze-Dried)
with DUPLOJECT System
TISSEEL Pre-Filled AST Syringe
(Frozen) with
DUO Set
TISSEEL Pre-Filled PRIMA Syringe (Frozen) with DUPLOJECT COMBI
2 mL
- 0338-4210-02
0338-4301-02
0338-8402-02
0338-9560-01
4 mL
- 0338-4211-04
0338-4302-04
0338-8402-04
0338-9564-01
10 mL
- 0338-4212-10
0338-4303-10
0338-8402-10
0338-9568-01
TISSEEL Kit contains one vial each of:
- 1.
- Sealer Protein Concentrate (Human), Vapor Heated, Solvent/Detergent Treated, Freeze-Dried, Sterile
- 2.
- Fibrinolysis Inhibitor Solution, (Synthetic) Liquid, Sterile
- 3.
- Thrombin (Human), Vapor Heated, Solvent/Detergent Treated, Freeze-Dried, Sterile
- 4.
- Calcium Chloride Solution, Liquid, Sterile
TISSEEL Pre-Filled Dual-Chambered Syringe contains:
- 1.
- Sealer Protein Solution, Vapor Heated, Solvent/Detergent Treated, Frozen Solution, Sterile
- 2.
- Thrombin Solution, Vapor Heated, Solvent/Detergent Treated, Frozen Solution, Sterile
- 3.
- Sterile accessory devices (DUO Set and Plunger or DUPLOJECT COMBI)
Storage and Handling
TISSEEL Kit (Freeze-Dried)
Store at 2-25°C. Avoid freezing. Do not freeze or refrigerate reconstituted solutions.TISSEEL Pre-filled Syringe (Frozen)
Store at ≤ -20°C. Do not refrigerate or re-freeze after thawing. Once removed from the freezer, TISSEEL must be used within 48 hours. Prior to application, TISSEEL must be warmed to 33 - 37˚C.
Once the pouches are opened or warmed to 33-37°C, they must be used within 4 hours.
Do not use after the expiration date. Discard if packaging of any components is damaged. -
17 PATIENT COUNSELING INFORMATION
Discuss the risks and benefits of this product with the patient since it is made from human plasma.
Instruct patients to consult their physician if symptoms of B19 virus infection appear (fever, drowsiness, chills and runny nose) followed about two weeks later by a rash and joint pain (see Use in Specific Populations (8.1)).
Manufactured For Baxter Healthcare Corporation
Deerfield, IL 60015 USA
US License No. 140Baxter, Artiss, Duo, Duploject, Duploject Combi, Duplospray, Easyspray, Fibrinotherm and Tisseel are trademarks of Baxter International Inc.
-
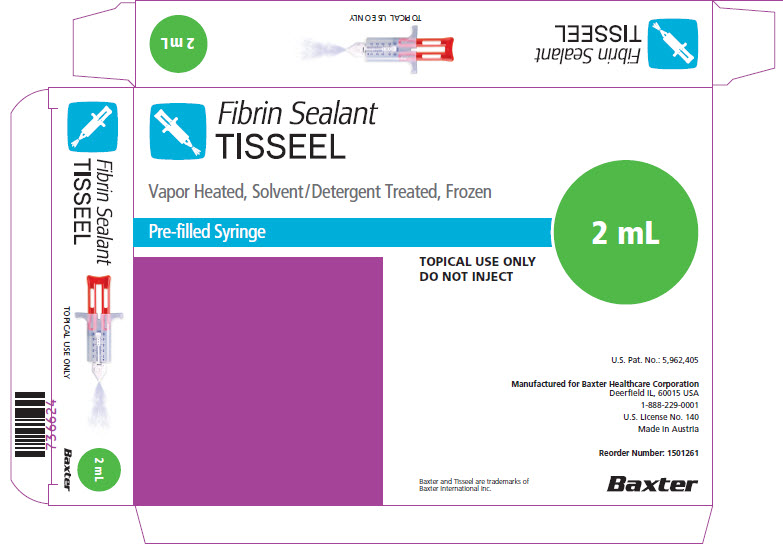
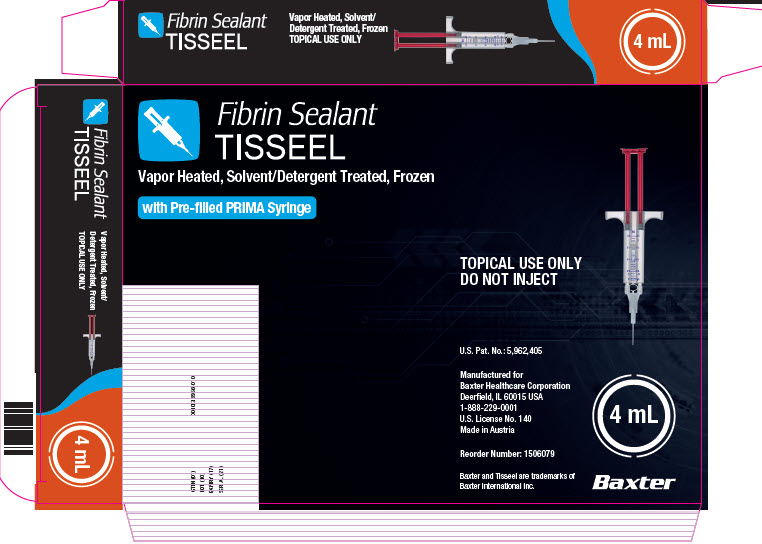
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Fibrin Sealant
TISSEEL 2 mLNDC 0338-8402-01
Vapor Heated, Solvent/Detergent Treated, Frozen
Baxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
TOPICAL USE ONLY DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches may be
stored for up to 48 hours at room temperature (15 – 25°C).Do not refrigerate or re-freeze.
Rx Only
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution (1): 1 mL, sterile
– Sealer Protein (Human)
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL– Thrombin Solution (2): 1 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 μmol/mLDMC
Manufactured for
Baxter Healthcare
Corporation
Deerfield, IL 60015 USAU.S. License No. 140
0736623Lot No.:
Exp. Date:
P
0736624
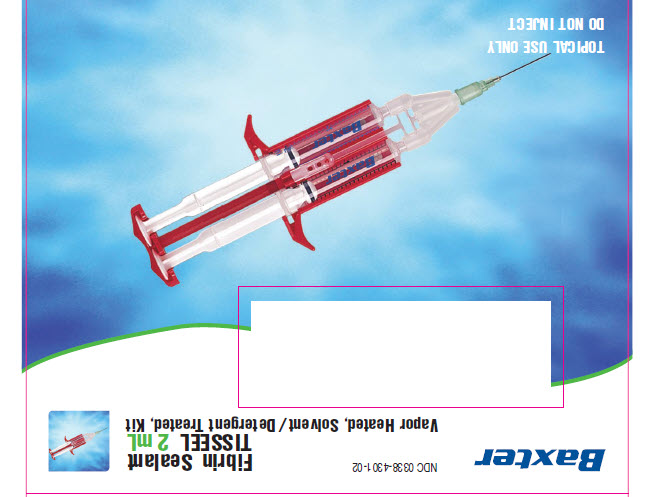
Fibrin Sealant
TISSEELVapor Heated, Solvent/Detergent Treated, Frozen
TOPICAL USE ONLY
2 mL
N3 0338-8402-02 3
Fibrin Sealant
TISSEELTOPICAL USE ONLY
2 mL
Fibrin Sealant
TISSEELVapor Heated, Solvent/Detergent Treated, Frozen
NDC 0338-8402-02
Prefilled Syringe
2 mL
TOPICAL USE ONLY
Contents:
Pre-filled syringe containing:Sealer Protein Solution: 1 mL, sterile
– Sealer Protein (Human)
– Fibrinolysis Inhibitor (Aprotinin, Synthetic), 3000 KIU/mLThrombin Solution: 1 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 μmol/mLThe risks and benefits of this product should be discussed with the patient.
Rx Only
DO NOT INJECT
Store at –20°C (–4°F) or colder.
Read enclosed directions for thawing and application before use.
Unopened pouches may be stored for up to 48 hours at room
temperature (15 – 25°C).Do not refrigerate or re-freeze.
Not made with natural rubber latex
For Single Use Only.
Baxter Logo
Fibrin Sealant
TISSEELTOPICAL USE ONLY
2 mL
Fibrin Sealant
TISSEELTOPICAL USE ONLY
2 mL
Baxter Logo
736624
Fibrin Sealant
TISSEELVapor Heated, Solvent/Detergent Treated, Frozen
Pre-filled Syringe
2 mL
TOPICAL USE ONLY
DO NOT INJECTU.S. Pat. No.: 5,962,405
Manufactured for Baxter Healthcare Corporation
Deerfield IL, 60015 USA
1-888-229-0001
U.S. License No. 140
Made in AustriaReorder Number: 1501261
Baxter and Tisseel are trademarks of
Baxter International Inc.Baxter Logo
NDC 0338-4210-02
Fibrin Sealant
TISSEEL 2 mL
Vapor Heated, Solvent/Detergent Treated, KitGTIN (01)
LOT (10)
EXPIRY (17)
SERIAL (21)Baxter Logo
NDC 0338-4210-02
Barcode
Fibrin Sealant
TISSEEL 2 mL
Vapor Heated, Solvent/Detergent Treated, KitContents:
Sealer Protein Concentrate (Human), Vapor Heated,
Solvent/Detergent Treated, freeze dried, sterile, for
1 mL of Sealer Protein Solution 86.5 mg/mLFibrinolysis Inhibitor Solution (Synthetic), sterile,
3000 KIU of Aprotinin /mLThrombin (Human), Vapor Heated, Solvent/Detergent
Treated, freeze dried, sterile, for 1 mL of Thrombin
Solution 500 units/mLCalcium Chloride Solution, sterile, 40 μmol /mL
The risks and benefits of this product should be discussed
with the patient.
Read enclosed directions for reconstitution and application
before use.
Use within four hours of reconstitution.NOT FOR INJECTION. For single use only.
Not made with natural rubber latex
Rx only
Manufactured for Baxter Healthcare Corporation
Deerfield, IL 60015 USAU.S. License No. 140
Made in Austria
U.S. Pat. No.: 5,962,405
Baxter and Tisseel are trademarks of Baxter International Inc.
0752668
Fibrin Sealant
TISSEEL 2 mL
Vapor Heated, Solvent/
Detergent Treated, Kit2 mL
Store at 2°C to 25°C (36°F to 77°F).
Avoid freezing.Baxter Logo
NDC 0338-4301-02
Fibrin Sealant
TISSEEL 2 mL
Vapor Heated, Solvent/Detergent Treated, KitManufactured for Baxter Healthcare Corporation
Deerfield, IL 60015 USAU.S. License No. 140
Made in Austria
Reorder Number: 1504514
Baxter, Duploject and Tisseel are trademarks of Baxter International Inc.
U.S. Pat. No.: 5,962,405
Baxter Logo
NDC 0338-4301-02
Fibrin Sealant
TISSEEL 2 mL
Vapor Heated, Solvent/Detergent Treated, KitTOPICAL USE ONLY
DO NOT INJECTBaxter Logo
NDC 0338-4301-02
Barcode
Fibrin Sealant
TISSEEL 2 mL
Vapor Heated, Solvent/Detergent Treated, KitContents:
Sealer Protein Concentrate (Human), Vapor Heated,
Solvent/Detergent Treated, freeze dried, sterile, for
1 mL of Sealer Protein Solution 86.5 mg/mLFibrinolysis Inhibitor Solution (Synthetic), sterile,
3000 KIU of Aprotinin /mLThrombin (Human), Vapor Heated, Solvent /Detergent
Treated, freeze dried, sterile, for 1 mL of Thrombin Solution
500 units/mLCalcium Chloride Solution, sterile, 40 μmol /mL
The risks and benefits of this product should be discussed
with the patient. Read enclosed directions for
reconstitution and application before use. Use within four
hours of reconstitution.NOT FOR INJECTION. For single use only.
Not made with natural rubber latex
Rx onlyAlso includes: DUPLOJECT Fibrin Sealant Preparation and Application System 2 mL / 4 mL
0752670
Barcode
Barcode
N3 0338-4301-02 3Baxter Label
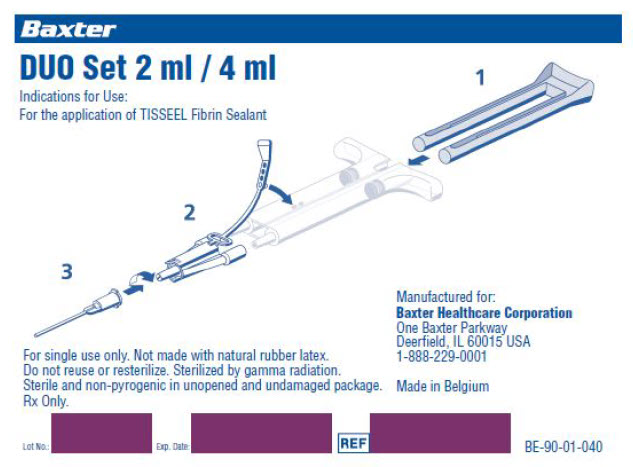
DUO Set 2 ml / 4ml
Indications for Use:
For the application of TISSEEL Fibrin Sealant
For single use only. Not made with natural rubber latex.
Do not reuse or resterilize. Sterilized by gamma radiation.
Sterile and non-pyrogenic in unopened and undamaged package.
Rx only.
Lot No:
Exp. Date:
REF
BE-90-01-040
Manufactured for:
Baxter Healthcare Corporation,
One Baxter Parkway
Deerfield, IL 60015 USAMade in Belgium
Baxter Logo
DUPLOJET 2 mL / 4 mL
Fibrin Sealant Preparation and
Application SystemBaxter Logo
DUPLOJET 2 mL / 4 mL
Fibrin Sealant Preparation and
Application SystemBAXTER, DUPLOJET and TISSEEL are trademarks of
Baxter International Inc., registered in the U.S. Patent and
Trademark office.Manufactured for:
Baxter Healthcare Corporation
One Baxter Parkway
Deerfield, IL 60015 USADUPLOJET 2 mL / 4 mL
Fibrin Sealant Preparation and
Application SystemFor Fibrin Sealant
2 mL or 4 mL Kit2 mL
4 mLIndications for Use:
For the preparation and application of TISSEEL
[Fibrin Sealant] 2 mL / 4 mL Kit.STERILE – SINGLE PATIENT USE ONLY – DO NOT RESTERILIZE
Use separate devices for reconstruction of Sealer Protein Concentrate
and Thrombin.Rx only
Made in Belgium
Barcode
BE8001137
BE-80-01-137
DUPLOJET 2 mL / 4 mL
Fibrin Sealant Preparation and
Application SystemFor Fibrin Sealant
2 mL or 4 mL Kit2 mL
4 mLBaxter Logo
BE_30-02-680DUPLOJECT
Fibrin Sealant Preparation and Application SystemIndications for Use:
For the preparation and application of TISSEEL [Fibrin Sealant] kit.
Note: See TISSEEL Fibrin Sealant package insert for additional preparation and
application instructions.1.0 Reconstitution Instructions for the Circulating Nurse (Using the FIBRINO-
THERM Heating and Stirring Device)1.1 Turn on the warming unit of the FIBRINOTHERM device (amber switch).
1.2 Open TISSEEL [Fibrin Sealant] kit and place all vials into the appropriately sized
heating wells of the FIBRINOTHERM device.1.3 Place the Sealer Protein vial into the large magnetic stirring well fitted with
the appropriately sized adapter ring (if necessary). Do not activate the stirring
mechanism at this time. The indicator light will remain lit until a temperature of
37°C (98.6°F) is reached. Allow several minutes for proper warming.1.4 Open Pack A of the DUPLOJECT Preparation and Application System and
assemble the blue-scaled and black-scaled syringes with the needles provided.1.5 Remove the flip-off caps from the blue-capped Sealer Protein Concentrate
and Fibrinolysis Inhibitor Solution vials and wipe with a non-iodine based dis-
infectant.1.6 Using the blue-scaled syringe, withdraw the Fibrinolysis Inhibitor Solution from
the vial, tilting the vial slightly to facilitate removal of all solution. Inject the
Fibrinolysis Inhibitor Solution into the Sealer Protein Concentrate vial. Gently
swirl the vial to ensure that the freeze-dried material is fully soaked. Note: Do not
invert or inject air into vials.1.7 Place the Sealer Protein Concentrate vial in the stirring well and activate the
stirring mechanism of the FIBRINOTHERM device (green switch). Reconstitution
is complete when no undissolved particles are visible. If Sealer Protein is not fully
dissolved after 20 minutes, discard and prepare a fresh kit. Continue with steps 8
through 11.1.8 Remove the flip-off caps from the black-capped Thrombin and Calcium Chloride
Solution vials and wipe with a non-iodine based disinfectant.1.9 Using the black-scaled syringe, withdraw the Calcium Chloride Solution from the
vial, tilting the vial slightly to facilitate removal of all solution. Inject the Calcium
Chloride Solution into the Thrombin vial. Note: Do not invert or inject air into vials.1.10 Swirl contents of the Thrombin vial briefly and return it to an appropriately sized
heating well in the FIBRINOTHERM device.1.11 Leave the Sealer Protein and Thrombin vials in the FIBRINOTHERM device until
the solutions are ready to be passed into the sterile field.2.0 Preparation Instructions for the Scrub Nurse
2.1 Open Pack B of the DUPLOJECT Preparation and Application System into the
sterile field.2.2 Open Packs 1, 2, and 3.
2.3 Assemble the blue-scaled and black-scaled syringes with the needles provided.
Note: For 5 mL DUPLOJECT systems, attach the larger needle to the blue-scaled
syringe.2.4 While the Circulating Nurse holds the vial slightly tilted, insert needle into
vial (bevel side down) and withdraw all of the Sealer Protein Solution into the
blue-scaled syringe using slow, constant aspiration. Discard needle in sharps
container.2.5 While the Circulating Nurse holds the vial slightly tilted, insert needle into vial
(bevel side down) and withdraw all of the Thrombin Solution into the black-scaled
syringe using slow, constant aspiration. Discard needle in sharps container.2.6 Remove any air bubbles from the syringes and ensure both syringes contain the
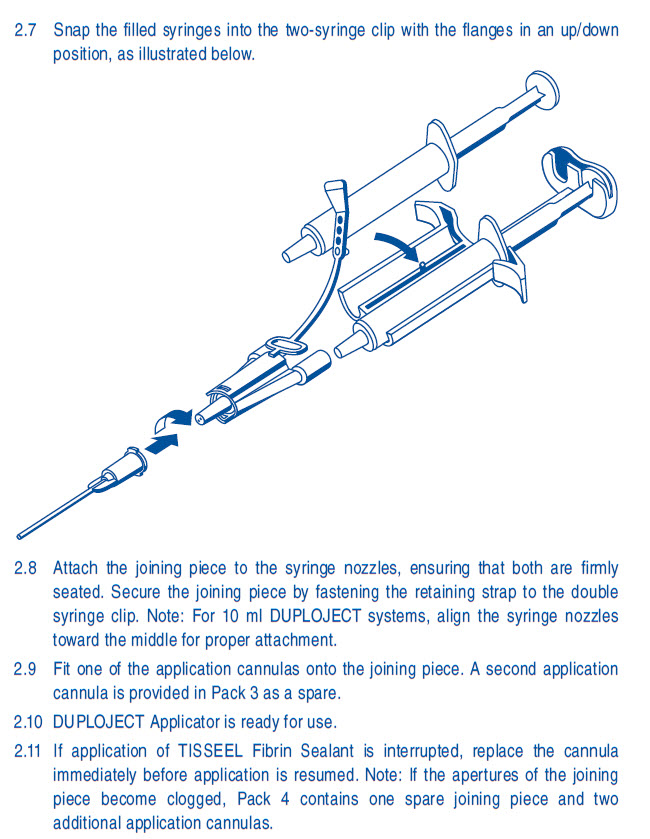
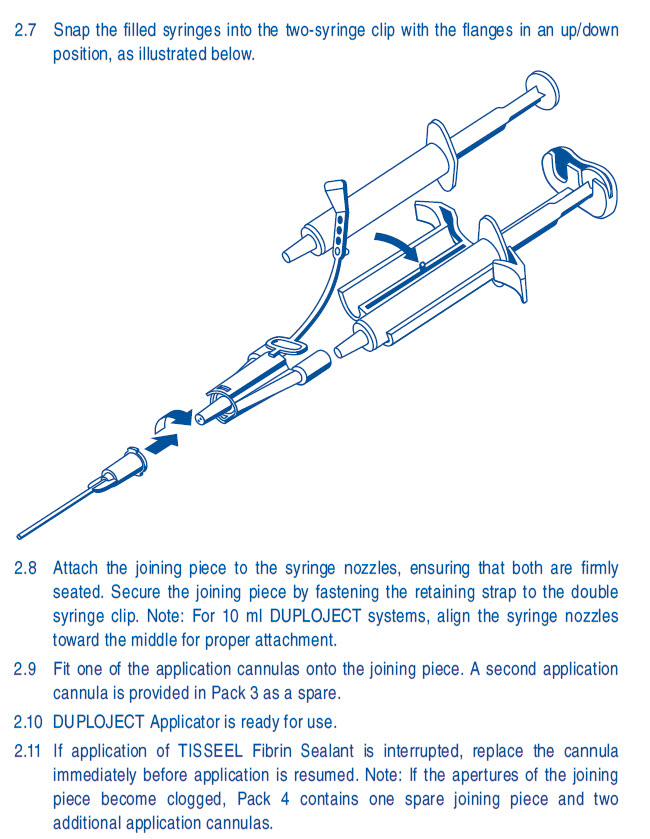
same volume.2.7 Snap the filled syringes into the two-syringe clip with the flanges in an up/down
position, as illustrated below.2.8 Attach the joining piece to the syringe nozzles, ensuring that both are firmly
seated. Secure the joining piece by fastening the retaining strap to the double
syringe clip. Note: Align the syringe nozzles toward the middle for proper
attachment.2.9 Fit one of the application needles onto the joining piece. A second application
needle is provided in Pack 3 as a spare.2.10 DUPLOJECT Applicator is ready for use.
2.11 If application of TISSEEL [Fibrin Sealant] is interrupted, replace the needle
immediately before application is resumed. Note: If the apertures of the joining
piece become clogged, Pack 4 contains one spare joining piece and two
additional application needles.Rx Only
DO NOT REUSE OR RESTERILIZE.
NOT MADE WITH NATURAL RUBBER LATEX.STERILE AND NON-PYROGENIC IN UNOPENED AND UNDAMAGED PACKAGE.
DISPOSE OF CONTAMINATED AND SHARP COMPONENTS PROPERLY.
Baxter, Duploject, Fibrinotherm and Tisseel are registered trademarks of Baxter International Inc.
Manufactured for:
Baxter Healthcare Corporation
One Baxter Parkway
Deerfield, IL 60015 USA
1-888-229-0001Made in Belgium
Rev. Date: 2017-05-17
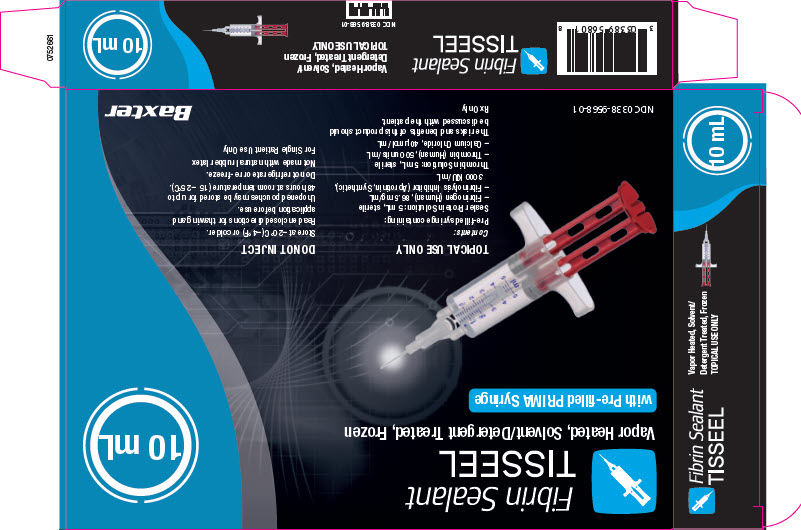
Fibrin Sealant
TISSEEL 2 mLNDC 0338-9560-01
Vapor Heated, Solvent/Detergent Treated, Frozen
Baxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
TOPICAL USE ONLY DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches
may be stored for up to 48 hours at room temperature
(15 – 25°C).Do not refrigerate or re-freeze.
Rx Only
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution (1): 1 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL– Thrombin Solution (2): 1 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 ìmol/mLManufactured for
Baxter Healthcare
Corporation
Deerfield, IL 60015 USA
U.S. License No. 140
0752652Barcode
Lot No.:
Exp. Date:
0752653
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLYNDC 0338-9560-01
BarcodeBarcode
3 03389 56001 22 mL
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/Detergent Treated, Frozen
with Pre-filled PRIMA SyringeTOPICAL USE ONLY
Contents:
Pre-filled syringe containing:
Sealer Protein Solution: 1 mL, sterile– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin, Synthetic),
3000 KIU/mLThrombin Solution: 1 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 μmol /mLThe risks and benefits of this product should
be discussed with the patient.Rx Only
DO NOT INJECT
Store at –20°C (–4°F) or colder.
Read enclosed directions for thawing and
application before use.Unopened pouches may be stored for up to
48 hours at room temperature (15 – 25°C).Do not refrigerate or re-freeze.
Not made with natural rubber latex
For Single Patient Use Only.NDC 0338-9560-01
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/Detergent Treated, Frozen
TOPICAL USE ONLY
2 mLFibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY
2 mLFibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY
2 mLFibrin Sealant
TISSEEL
Vapor Heated, Solvent/Detergent Treated, Frozen
with Pre-filled PRIMA SyringeTOPICAL USE ONLY
DO NOT INJECT
U.S. Pat. No.: 5,962,405
Manufactured for
Baxter Healthcare CorporationDeerfield IL, 60015 USA
1-888-229-0001
U.S. License No. 140
Made in Austria
Reorder Number: 1506078
Baxter and Tisseel are trademarks of
Baxter International Inc.2 mL
GTIN (01) 00303389560012
EXPIRY (17)
LOT (10)
SERIAL (21)Baxter Logo
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY
2 mLFibrin Sealant
TISSEEL 4 mLNDC 0338-9564-01
BarcodeVapor Heated, Solvent/Detergent Treated, Frozen
Baxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
TOPICAL USE ONLY DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches
may be stored for up to 48 hours at room temperature
(15 – 25°C).Do not refrigerate or re-freeze.
Rx Only
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution (1): 2 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL– Thrombin Solution (2): 2 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 µmol/mLBarcode
Manufactured for
Baxter Healthcare
Corporation
Deerfield, IL 60015 USA
U.S. License No. 1400752656
Barcode
Lot No.:
Exp. Date:0752657
Barcode
3 03389 56401 0Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/Detergent Treated, Frozen
TOPICAL USE ONLY
with Pre-filled PRIMA SyringeNDC 0338-9564-01
TOPICAL USE ONLY
Contents:
Pre-filled syringe containing:
Sealer Protein Solution: 2 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin, Synthetic),
3000 KIU/mLThrombin Solution: 2 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 μmol /mLThe risks and benefits of this product should
be discussed with the patient.Rx Only
DO NOT INJECT
Store at –20°C (–4°F) or colder.
Read enclosed directions for thawing and
application before use.Unopened pouches may be stored for up to
48 hours at room temperature (15 – 25°C).Do not refrigerate or re-freeze.
Not made with natural rubber latex
For Single Patient Use Only.Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY
4 mLFibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY
4 mLFibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY
4 mLFibrin Sealant
TISSEEL
Vapor Heated, Solvent/Detergent Treated, Frozen
with Pre-filled PRIMA SyringeTOPICAL USE ONLY
DO NOT INJECT
U.S. Pat. No.: 5,962,405
Manufactured for
Baxter Healthcare CorporationDeerfield IL, 60015 USA
1-888-229-0001
U.S. License No. 140
Made in Austria
Reorder Number: 1506079
Baxter and Tisseel are trademarks of
Baxter International Inc.4 mL
Baxter Logo
GTIN (01) 00303389564010
LOT (10)
EXPIRY (17)
SERIAL (21)Fibrin Sealant
TISSEEL 10 mLNDC 0338-9568-01
Vapor Heated, Solvent/Detergent Treated, Frozen
Baxter Logo
Temperature sensitive – Do NOT expose above 37°C (99°F).
TOPICAL USE ONLY DO NOT INJECT
Read directions for thawing and application before use.
Store at –20°C (–4°F) or colder. Unopened pouches
may be stored for up to 48 hours at room temperature
(15 – 25°C).Do not refrigerate or re-freeze.
Rx Only
Contents:
Pre-filled syringe containing:
– Sealer Protein Solution (1): 5 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin,
Synthetic), 3000 KIU/mL– Thrombin Solution (2): 5 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 ìmol/mLManufactured for
Baxter Healthcare
Corporation
Deerfield, IL 60015 USA
U.S. License No. 1400752660
Barcode
Barcode
Lot No.:
Exp. Date:0752661
Barcode
3 03389 56801 8Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLYNDC 0338-9568-01
Barcode10 mL
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY10 mL
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/Detergent Treated, Frozen
with Pre-filled PRIMA SyringeTOPICAL USE ONLY
Contents:
Pre-filled syringe containing:
Sealer Protein Solution: 5 mL, sterile
– Fibrinogen (Human), 86.5 mg/mL
– Fibrinolysis Inhibitor (Aprotinin, Synthetic),
3000 KIU/mLThrombin Solution: 5 mL, sterile
– Thrombin (Human), 500 units/mL
– Calcium Chloride, 40 μmol /mLThe risks and benefits of this product should
be discussed with the patient.Rx Only
DO NOT INJECT
Store at –20°C (–4°F) or colder.
Read enclosed directions for thawing and
application before use.Unopened pouches may be stored for up to
48 hours at room temperature (15 – 25°C).Do not refrigerate or re-freeze.
Not made with natural rubber latex
For Single Patient Use Only
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY10 mL
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/
Detergent Treated, Frozen
TOPICAL USE ONLY
10 mLBarcode
Fibrin Sealant
TISSEEL
Vapor Heated, Solvent/Detergent Treated, Frozen
with Pre-filled PRIMA SyringeTOPICAL USE ONLY
DO NOT INJECT
U.S. Pat. No.: 5,962,405
Manufactured for
Baxter Healthcare CorporationDeerfield IL, 60015 USA
1-888-229-0001
U.S. License No. 140
Made in Austria
Reorder Number: 1506080
Baxter and Tisseel are trademarks of
Baxter International Inc.10 mL
Baxter Logo
GTIN (01) 00303389568018
EXPIRY (17)
LOT (10)
SERIAL (21)GTIN (01)
LOT (10)
EXPIRY (17)SERIAL (21)
NDC 0338-4211-04
Fibrin Sealant
TISSEEL 4 mL
Vapor Heated, Solvent/Detergent Treated, KitBaxter logo
NDC 0338-4211-04
barcodeFibrin Sealant
TISSEEL 4 mL
Vapor Heated, Solvent/Detergent Treated, KitContents:
Sealer Protein Concentrate (Human), Vapor Heated,
Solvent /Detergent Treated, freeze dried, sterile, for
2 mL of Sealer Protein Solution
Fibrinolysis Inhibitor Solution (Synthetic), sterile,
3000 KIU of Aprotinin /mL
Thrombin (Human), Vapor Heated, Solvent /Detergent
Treated, freeze dried, sterile, for 2 mL of Thrombin
Solution 500 units/mL
Calcium Chloride Solution, sterile, 40 μmol /mLThe risks and benefits of this product should be discussed
with the patient.
Read enclosed directions for reconstitution and application
before use.
Use within four hours of reconstitution.
NOT FOR INJECTION. For single use only.
Not made with natural rubber latex
Rx onlyManufactured for Baxter Healthcare Corporation
Deerfield, IL 60015 USAU.S. License No. 140
Made in Austria
Baxter and Tisseel are trademarks of Baxter International Inc.
U.S. Pat. No.: 5,962,405
Barcode
0752678Fibrin Sealant
TISSEEL 4 mL
Vapor Heated, Solvent/
Detergent Treated, Kit4 mL
Store at 2°C to 25°C (36°F to 77°F).
Avoid freezing.GTIN (01)
LOT (10)
EXPIRY (17)SERIAL (21)
NDC 0338-4212-10
Fibrin Sealant
TISSEEL 10 mL
Vapor Heated, Solvent/Detergent Treated, KitBaxter Logo
NDC 0338-4212-10
barcodeFibrin Sealant
TISSEEL 10 mL
Vapor Heated, Solvent/Detergent Treated, KitContents:
Sealer Protein Concentrate (Human), Vapor Heated,
Solvent /Detergent Treated, freeze dried, sterile, for
5 mL of Sealer Protein Solution 86.5 mg/mLFibrinolysis Inhibitor Solution (Synthetic), sterile,
3000 KIU of Aprotinin /mLThrombin (Human), Vapor Heated, Solvent /Detergent
Treated, freeze dried, sterile, for 5 mL of Thrombin
Solution 500 units/mLCalcium Chloride Solution, sterile, 40 µmol /mL
The risks and benefits of this product should be discussed
with the patient.
Read enclosed directions for reconstitution and application
before use.
Use within four hours of reconstitution.NOT FOR INJECTION. For single use only.
Not made with natural rubber latex
Rx only
Manufactured for Baxter Healthcare Corporation
Deerfield, IL 60015 USAU.S. License No. 140
Made in Austria
Baxter and Tisseel are trademarks of Baxter International Inc.
U.S. Pat. No.: 5,962,405
Barcode
0752688
Fibrin Sealant
TISSEEL 10 mL
Vapor Heated, Solvent/
Detergent Treated, Kit10 mL
Store at 2°C to 25°C (36°F to 77°F).
Avoid freezing.Baxter Logo
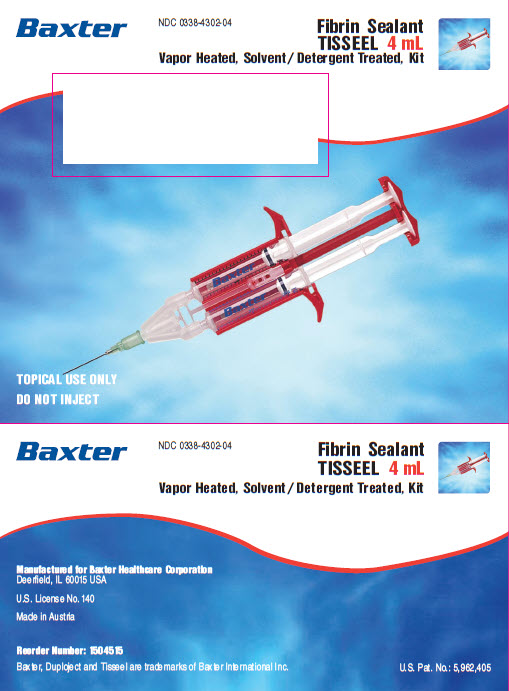
NDC 0338-4302-04
Fibrin Sealant
TISSEEL 4 mL
Vapor Heated, Solvent/Detergent Treated, KitManufactured for Baxter Healthcare Corporation
Deerfield, IL 60015 USAU.S. License No. 140
Made in Austria
Reorder Number: 1504515
Baxter, Duploject and Tisseel are trademarks of Baxter International Inc.
U.S. Pat. No.: 5,962,405
Baxter Logo
NDC 0338-4302-04
Fibrin Sealant
TISSEEL 4 mL
Vapor Heated, Solvent/Detergent Treated, KitTOPICAL USE ONLY
DO NOT INJECTBaxter Logo
NDC 0338-4302-04
barcodeFibrin Sealant
TISSEEL 4 mL
Vapor Heated, Solvent/Detergent Treated, KitContents:
Sealer Protein Concentrate (Human), Vapor Heated,
Solvent /Detergent Treated, freeze dried, sterile, for
2 mL of Sealer Protein Solution 86.5 mg/mLFibrinolysis Inhibitor Solution (Synthetic), sterile,
3000 KIU of Aprotinin /mLThrombin (Human), Vapor Heated, Solvent /Detergent
Treated, freeze dried, sterile, for 2 mL of Thrombin Solution
500 units/mLCalcium Chloride Solution, sterile, 40 μmol /mL
The risks and benefits of this product should be discussed
with the patient. Read enclosed directions for
reconstitution and application before use. Use within four
hours of reconstitution.NOT FOR INJECTION. For single use only.
Not made with natural rubber latex
Rx only
Also includes: DUPLOJECT Fibrin Sealant Preparation and Application System 2 mL / 4 mL
0752680
Barcode
Barcode
N3 0338-4302-04 4Baxter Logo
NDC 0338-4303-10
Fibrin Sealant
TISSEEL 10 mL
Vapor Heated, Solvent/Detergent Treated, KitManufactured for Baxter Healthcare Corporation
Deerfield, IL 60015 USAU.S. License No. 140
Made in Austria
Reorder Number: 1504516
Baxter, Duploject and Tisseel are trademarks of Baxter International Inc.
U.S. Pat. No.: 5,962,405
Baxter Logo
NDC 0338-4303-10
Fibrin Sealant
TISSEEL 10 mL
Vapor Heated, Solvent/Detergent Treated, KitTOPICAL USE ONLY
DO NOT INJECTBaxter Logo
NDC 0338-4303-10
Barcode
Fibrin Sealant
TISSEEL 10 mL
Vapor Heated, Solvent/Detergent Treated, KitContents:
Sealer Protein Concentrate (Human), Vapor Heated,
Solvent /Detergent Treated, freeze dried, sterile, for
5 mL of Sealer Protein Solution 86.5 mg/mLFibrinolysis Inhibitor Solution (Synthetic), sterile,
3000 KIU of Aprotinin /mLThrombin (Human), Vapor Heated, Solvent /Detergent
Treated, freeze dried, sterile, for 5 mL of Thrombin Solution
500 units/mLCalcium Chloride Solution, sterile, 40 μmol /mL
The risks and benefits of this product should be discussed
with the patient. Read enclosed directions for
reconstitution and application before use. Use within four
hours of reconstitution.
NOT FOR INJECTION. For single use only.
Not made with natural rubber latex
Rx onlyAlso includes: DUPLOJECT Fibrin Sealant Preparation and Application System 10 mL
0752690
Barcode
Barcode
N3 0338-4303-10 2Baxter Logo
DuploJect Combi
Indication for Use:
For the application of TISSEEL Fibrin Sealant.REF
Batch code
Use-by dateFor single use only. Not made with natural rubber latex.
Do not reuse or resterilize. Sterilized using ethylene oxide.
Sterile and non-pyrogenic in unopened and undamaged package. Rx Only.Manufactured for:
Baxter Healthcare Corporation
One Baxter Parkway
Deerfield, IL 60015 USA
1-888-229-0001Made in Belgium
BE-90-01-044
-
INGREDIENTS AND APPEARANCE
TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-4210 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-4210-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 1 mL Part 2 1 VIAL, GLASS 1 mL Part 3 1 VIAL, GLASS 1 mL Part 4 1 VIAL, GLASS 1 mL Part 1 of 4 SEALER PROTEIN CONCENTRATE HUMAN
fibrinogen human powder, for solutionProduct Information Item Code (Source) NDC:0338-7112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7112-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 2 of 4 HUMAN THROMBIN
human thrombin powder, for solutionProduct Information Item Code (Source) NDC:0338-7332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7332-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 3 of 4 FIBRINOLYSIS INHIBITOR SOLUTION
aprotinin liquidProduct Information Item Code (Source) NDC:0338-7201 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) 3000 [iU] in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7201-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 4 of 4 CALCIUM CHLORIDE SOLUTION
calcium chloride liquidProduct Information Item Code (Source) NDC:0338-7401 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 40 umol in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7401-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-4211 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-4211-04 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 2 mL Part 2 1 VIAL, GLASS 2 mL Part 3 1 VIAL, GLASS 2 mL Part 4 1 VIAL, GLASS 2 mL Part 1 of 4 SEALER PROTEIN CONCENTRATE HUMAN
fibrinogen human powder, for solutionProduct Information Item Code (Source) NDC:0338-7112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7112-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 2 of 4 HUMAN THROMBIN
human thrombin powder, for solutionProduct Information Item Code (Source) NDC:0338-7332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7332-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 3 of 4 FIBRINOLYSIS INHIBITOR SOLUTION
aprotinin liquidProduct Information Item Code (Source) NDC:0338-7201 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) 3000 [iU] in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7201-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 4 of 4 CALCIUM CHLORIDE SOLUTION
calcium chloride liquidProduct Information Item Code (Source) NDC:0338-7401 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 40 umol in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7401-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-4212 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-4212-10 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 3 1 VIAL, GLASS 5 mL Part 4 1 VIAL, GLASS 5 mL Part 1 of 4 SEALER PROTEIN CONCENTRATE HUMAN
fibrinogen human powder, for solutionProduct Information Item Code (Source) NDC:0338-7112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7112-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 2 of 4 HUMAN THROMBIN
human thrombin powder, for solutionProduct Information Item Code (Source) NDC:0338-7332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7332-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 3 of 4 FIBRINOLYSIS INHIBITOR SOLUTION
aprotinin liquidProduct Information Item Code (Source) NDC:0338-7201 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) 3000 [iU] in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7201-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 4 of 4 CALCIUM CHLORIDE SOLUTION
calcium chloride liquidProduct Information Item Code (Source) NDC:0338-7401 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 40 umol in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7401-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-4301 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-4301-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 1 mL Part 2 1 VIAL, GLASS 1 mL Part 3 1 VIAL, GLASS 1 mL Part 4 1 VIAL, GLASS 1 mL Part 1 of 4 SEALER PROTEIN CONCENTRATE HUMAN
fibrinogen human powder, for solutionProduct Information Item Code (Source) NDC:0338-7112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7112-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 2 of 4 HUMAN THROMBIN
human thrombin powder, for solutionProduct Information Item Code (Source) NDC:0338-7332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7332-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 3 of 4 FIBRINOLYSIS INHIBITOR SOLUTION
aprotinin liquidProduct Information Item Code (Source) NDC:0338-7201 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) 3000 [iU] in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7201-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 4 of 4 CALCIUM CHLORIDE SOLUTION
calcium chloride liquidProduct Information Item Code (Source) NDC:0338-7401 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 40 umol in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7401-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-4302 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-4302-04 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 2 mL Part 2 1 VIAL, GLASS 2 mL Part 3 1 VIAL, GLASS 2 mL Part 4 1 VIAL, GLASS 2 mL Part 1 of 4 SEALER PROTEIN CONCENTRATE HUMAN
fibrinogen human powder, for solutionProduct Information Item Code (Source) NDC:0338-7112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7112-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 2 of 4 HUMAN THROMBIN
human thrombin powder, for solutionProduct Information Item Code (Source) NDC:0338-7332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7332-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 3 of 4 FIBRINOLYSIS INHIBITOR SOLUTION
aprotinin liquidProduct Information Item Code (Source) NDC:0338-7201 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) 3000 [iU] in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7201-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 4 of 4 CALCIUM CHLORIDE SOLUTION
calcium chloride liquidProduct Information Item Code (Source) NDC:0338-7401 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 40 umol in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7401-02 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-4303 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-4303-10 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 3 1 VIAL, GLASS 5 mL Part 4 1 VIAL, GLASS 5 mL Part 1 of 4 SEALER PROTEIN CONCENTRATE HUMAN
fibrinogen human powder, for solutionProduct Information Item Code (Source) NDC:0338-7112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7112-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 2 of 4 HUMAN THROMBIN
human thrombin powder, for solutionProduct Information Item Code (Source) NDC:0338-7332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7332-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 3 of 4 FIBRINOLYSIS INHIBITOR SOLUTION
aprotinin liquidProduct Information Item Code (Source) NDC:0338-7201 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) 3000 [iU] in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7201-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Part 4 of 4 CALCIUM CHLORIDE SOLUTION
calcium chloride liquidProduct Information Item Code (Source) NDC:0338-7401 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 40 umol in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-7401-05 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-8402 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 90 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-8402-02 1 in 1 CARTON 1 NDC:0338-8402-01 2 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0338-8402-04 1 in 1 CARTON 2 NDC:0338-8402-03 4 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 3 NDC:0338-8402-10 1 in 1 CARTON 3 NDC:0338-8402-09 10 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-9560 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9560-01 2 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-9564 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9564-01 4 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 TISSEEL FIBRIN SEALANT
fibrinogen human, human thrombin solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0338-9568 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIBRINOGEN HUMAN (UNII: N94833051K) (FIBRINOGEN HUMAN - UNII:N94833051K) FIBRINOGEN HUMAN 86.5 mg in 1 mL HUMAN THROMBIN (UNII: 6K15ABL77G) (HUMAN THROMBIN - UNII:6K15ABL77G) HUMAN THROMBIN 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength APROTININ (UNII: 04XPW8C0FL) ALBUMIN HUMAN (UNII: ZIF514RVZR) HISTIDINE (UNII: 4QD397987E) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0338-9568-01 10 mL in 1 SYRINGE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103980 05/01/1998 Labeler - Baxter Healthcare Corporation (005083209)