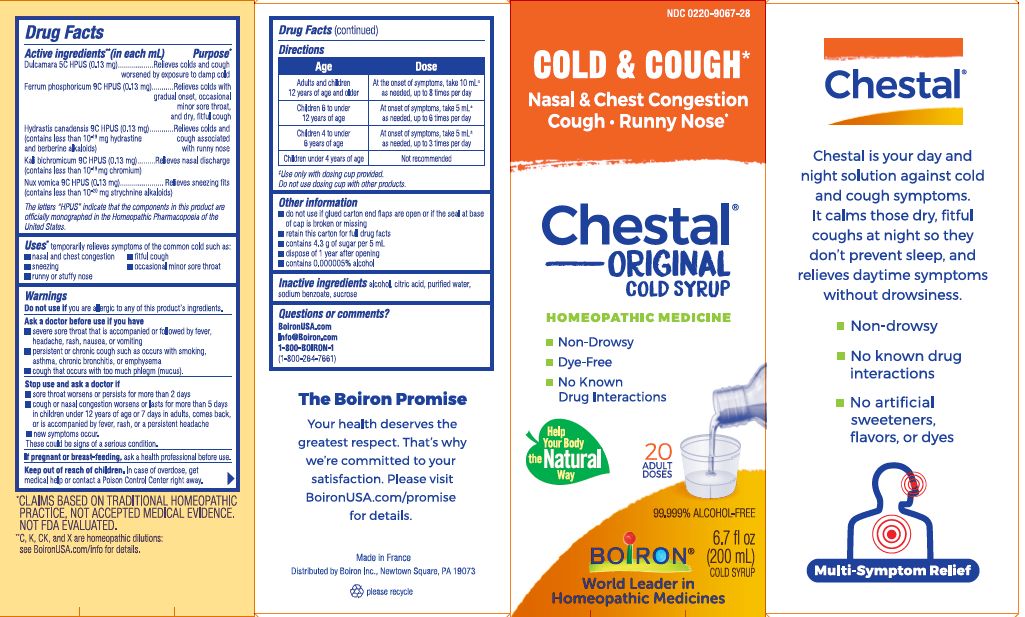
Label: CHESTAL COUGH AND COLD- solanum dulcamara top, ferrum phosphoricum, goldenseal, potassium dichromate, strychnos nux-vomica seed syrup
- NDC Code(s): 0220-9067-28
- Packager: Laboratoires Boiron
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients** (in each mL)
Dulcamara 5C HPUS (0.13 mg)
Ferrum phosphoricum 9C HPUS (0.13 mg)
Hydrastis canadensis 9C HPUS (0.13 mg) (contains less than 10- 19 mg hydrastine and berberine alkaloids)
Kali bichromicum 9C HPUS (0.13mg) (contains less than 10 -19 mg chromium)
Nux vomica 9C HPUS (0.13mg) (contains less than 10 -20 mg strychnine alkaloids)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
-
PURPOSE
Purpose*
Dulcamara 5C HPUS (0.13mg) ... Relieves cold and coughs worsened by exposure to damp cold
Ferrum phosphoricum 9C HPUS (0.13mg) ... Relieves colds with gradual onset, minor sore throat, and dry, fitful cough
Hydrastis canadensis 9C HPUS (0.13mg) ... Relieves colds and coughs associated with runny nose
Kali bichromicum 9C HPUS (0.13mg) ... Relieves nasal discharge
Nux vomica 9C HPUS (0.13mg) ... Relieves sneezing fits
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
-
STOP USE
Stop use and ask a doctor if
- sore throat worsens or persists for more than 2 days
- cough or nasal congestion worsens or lasts for more than 5 days in children under 12 years of age or 7 days in adults, comes back, or is accompanied by fever, rash, or a persistent headache
- new symptoms occur.
These could be signs of a serious condition.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Age Adults and children 12 years of age and older Dose At the onset of symptoms, take 10 mL ± as needed, up to 8 times per day
Age Children 6 to under 12 years of age Dose At the onset of symptoms, take 5 mL ± as needed, up to 6 times per day
Age Children 4 to under 6 years of age Dose At the onset of symptoms, take 5 mL ± as needed, up to 3 times per day
Age Children under 4 years of age Dose Not recommended
±Use only with dosing cup provided.
-
SPL UNCLASSIFIED SECTION
do not use if glued carton end flaps are open or if the seal at base of cap is broken or missing
retain this carton for full drug facts
contains 4.3 g of sugar per 5 mL
dispose of 1 year after opening
contains 0.000005% alcohol
Multi-symptom Cold & Cough Relief*
Cold & Cough Relief*
Nasal & Chest Congestion Cough, Runny Nose*
Non-Drowsy
Dye-Free
No Known Drug Interactions
6.7 fl. oz. (200 mL) cold syrup
20 Adult Doses
99.999% ALCOHOL FREE
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details. - INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHESTAL COUGH AND COLD
solanum dulcamara top, ferrum phosphoricum, goldenseal, potassium dichromate, strychnos nux-vomica seed syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9067 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 5 [hp_C] in 1 mL FERRUM PHOSPHORICUM (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERRUM PHOSPHORICUM 9 [hp_C] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 9 [hp_C] in 1 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 9 [hp_C] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 9 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCROSE (UNII: C151H8M554) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9067-28 1 in 1 PACKAGE 06/01/2013 1 200 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2013 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9067)