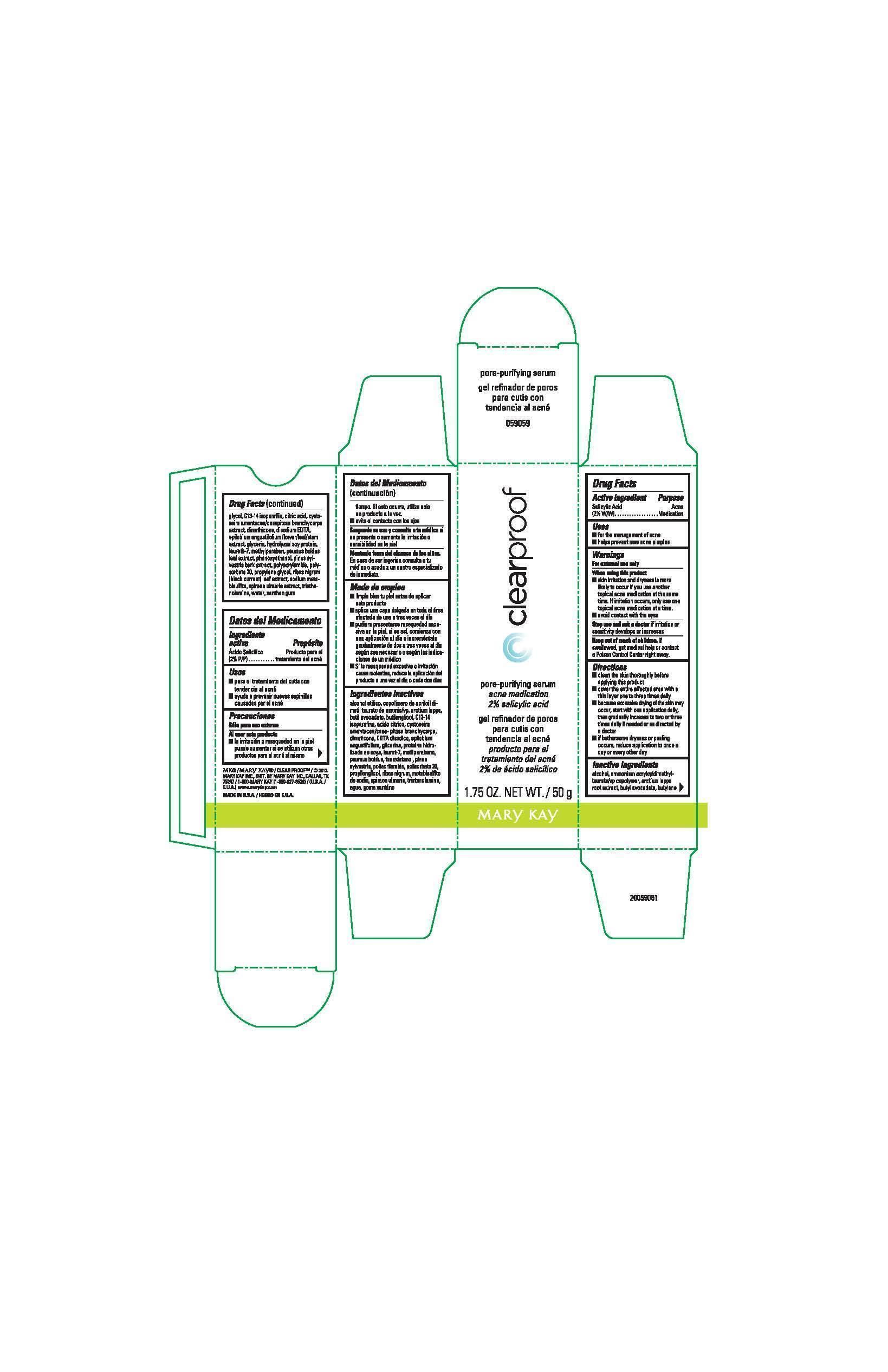
CLEAR PROOF PORE PURIFYING SERUM ACNE MEDICATION- salicylic acid solution
Mary Kay Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic Acid (2% W/W)
Uses
- for the management of acne
- helps prevent new acne pimples
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid contact with the eyes
Stop use and ask a doctor
if irritation or sensitivity develops or increases
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
alcohol, ammonium acryloyldimethyltaurate/vp copolymer, arctium lappa root extract, butyl avocadate, butylene glycol, C13-14 isoparaffin, citric acid, cystoseira amentacea/caespitosa branchycarpa extract, dimethicone, disodium EDTA, epilobium angustifolium flower/leaf/stem extract, glycerin, hydrolyzed soy protein, laureth-7, methylparaben, peumus boldus leaf extract, phenoxyethanol, pinus sylvestris bark extract, polyacrylamide, polysorbate 20, propylene glycol, ribes nigrum (black currant) leaf extract, sodium metabisulfite, spiraea ulmaria extract, triethanolamine, water, xanthan gum
Principal Display Panel - 50 g carton
clearproof
pore-purifying serum
acne medication
2% salicylic acid
1.75 OZ. NET WT. / 50 g
Mary Kay
Mary Kay Inc.
