ELIXICURE LAVENDER WITH CBD- menthol, camphor, cannabidiol cream
Honest Globe Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
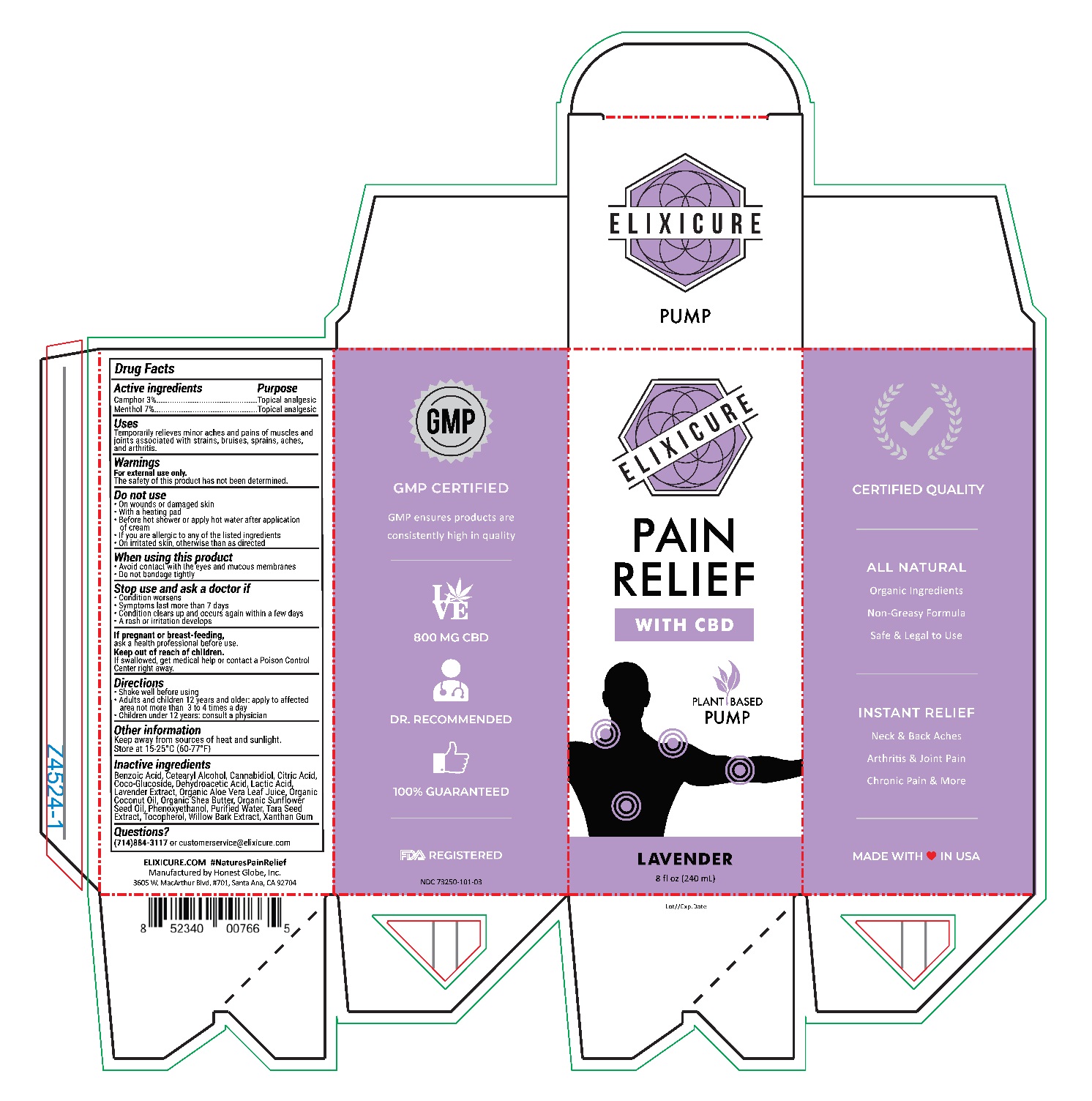
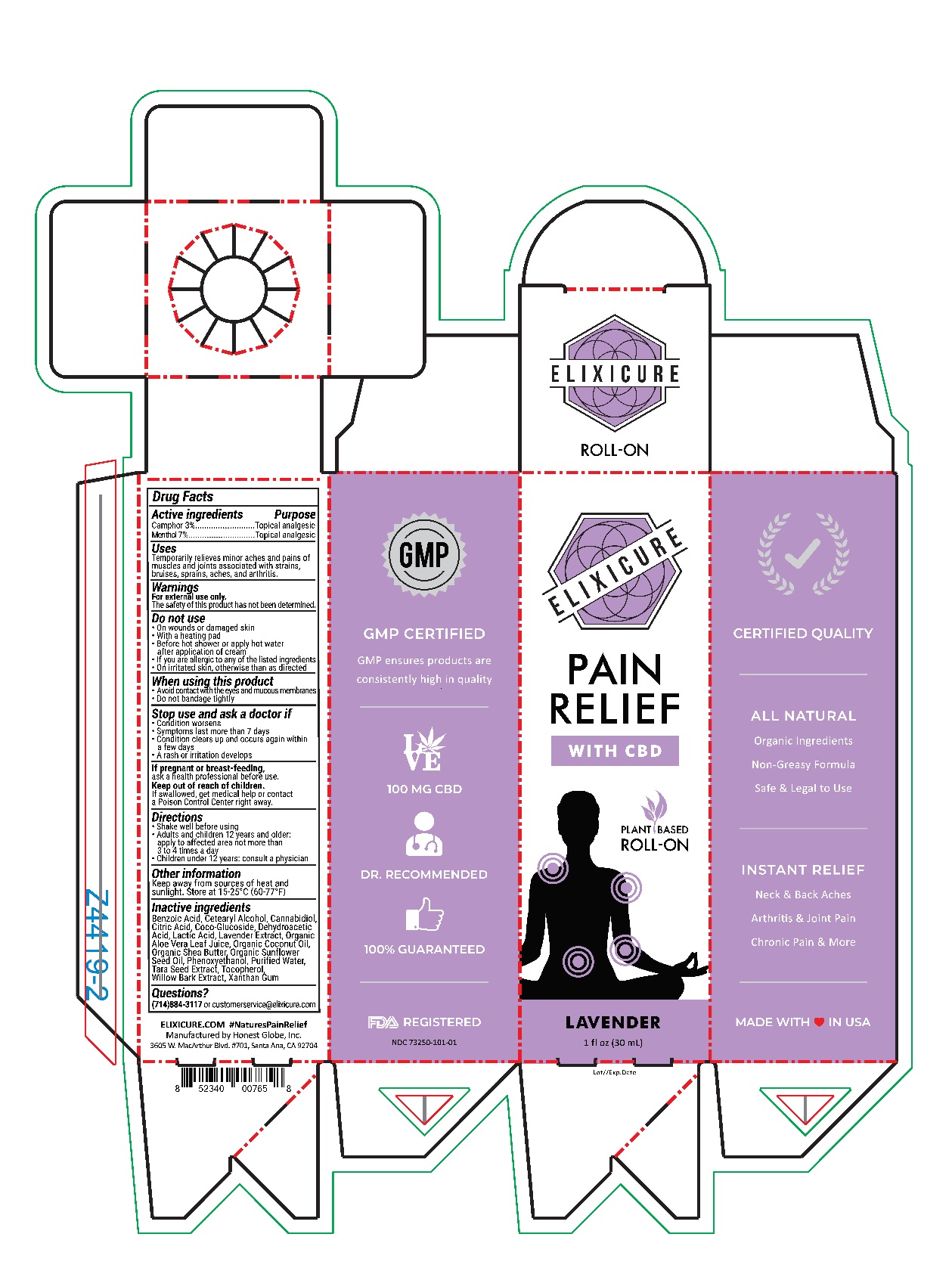
Elixicure Lavender with CBD
Uses
Temporarily relieves minor aches and pains of muscles and joints associated with strains, bruises, sprains, aches, and arthritis.
Do Not Use
On wounds or damaged skin
With a heating pad
Before hot shower or apply hot water after application of cream
If you are allergic to any of the listed ingredients
On irritated skin, otherwise than as directed
Stop use and ask a doctor if
Condition worsens
Symptoms last more than 7 days
Condition clears up and occurs again within a few days
A rash or irritation develops
Directions
Shake well before using
Adults and Children 12 years or older: Apply to affected area not more than 3-4 times a day
Children under age of 12: Consult a physician
Inactive Ingredients
Benzoic Acid, Cetearyl Alcohol, Cannabidiol, Citric Acid, Coco-Glucoside, Dehydroacetic Acid, Lactic Acid, Lacender Extract, Organic Aloe Vera Leaf Juice, Organic Coconut Oil, Organic Shea Butter, Organic Sunflower Seed Oil, Phenoxyethanol, Purified Water, Tara Seed Extract, Tocopherol, Willow Bark Extract, Xanthan Gum
| ELIXICURE LAVENDER WITH CBD
menthol, camphor, cannabidiol cream |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Honest Globe Inc (051811972) |