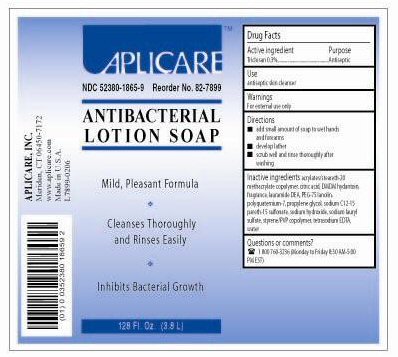
ANTIBACTERIAL- triclosan liquid
Aplicare Products, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
1865 Antibacterial
Add small amount of soap to wet hands and forearms.
Develop lather.
Scrub well and rinse thoroughly after washing.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
| ANTIBACTERIAL
triclosan liquid |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Aplicare Products, LLC. (081054904) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare Products, LLC. | 081054904 | manufacture(52380-1865) | |
Revised: 1/2020
Document Id: 9d4d66f7-fa47-e832-e053-2a95a90a8b62
Set id: b24b2229-cf04-4a01-8c9f-7d823f501d75
Version: 3
Effective Time: 20200129
Aplicare Products, LLC.