ABEE MED- menthol, histamine dihydrochloride cream
Crossover Telecom Llc
----------
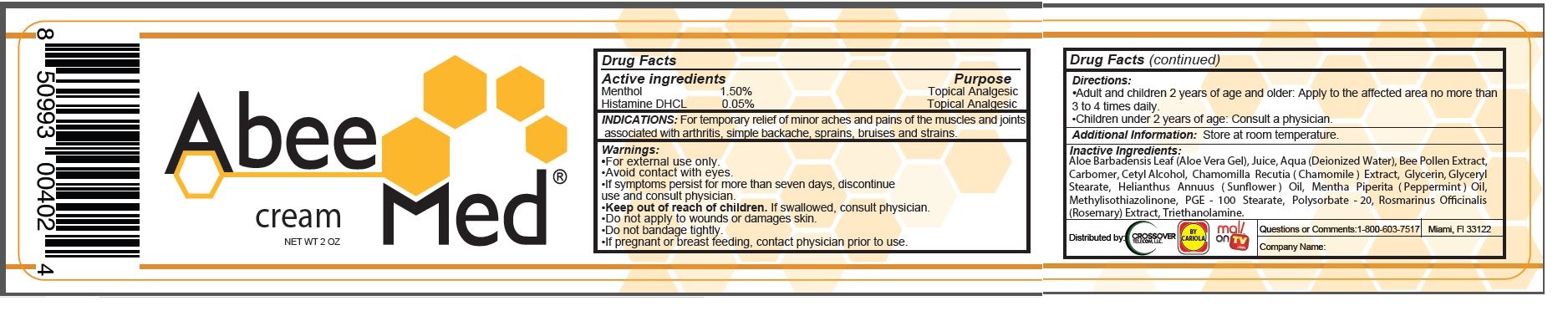
Abee Med Cream
INDICATIONS:
For temporary relief of minor aches and pains of the muscles and joints associated with arthritis, simple backache, sprains, bruises and strains.
Warnings:
- For external use only.
- Avoid contact with eyes.
- If symptoms persist for more than seven days, discontinue use and consult physician.
Directions:
- Adult and children 2 years fo age and older: Apply to the affected area on more than 3 to 4 times daily.
- Children under 2 years of age: Consult a physician.
Inacitve Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel), Juice, Aqua (Deionized Water), Bee Pollen Extract, Carbomer, Cetyl Alcohol, Chamomilla Recutia (Chamomile) Extract, Glycerin, Glyceryl Stearate, Helianthus Annuus (Sunflower) Oil, Mentha Piperita (Peppermint) Oil, Methylisothiazolinone, PGE - 100 Stearate, Polysorbate - 20, Rosmarinus Officinalis (Rosemary) Extract, Triethanolamine.
| ABEE MED
menthol, histamine dihydrochloride cream |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Crossover Telecom Llc (026911787) |
Revised: 10/2023
Document Id: 071d7488-7a7d-5a60-e063-6394a90a0f5d
Set id: b23f0b78-1617-4e39-9c0f-fd985c7f55d5
Version: 4
Effective Time: 20231007
Crossover Telecom Llc