Label: VISTA ADVANCED CAROTENOID FORMULA capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 77790-007-12 - Packager: Red Wedding LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- VISTA ADVANCED TM
-
INDICATIONS & USAGE
Suggested Use:
Take 1 softgel twice daily, one in the morning and one in the evening with meals for best results.
- Maintains and supports retinal function‡
- Reduces oxidation‡
- Supports vision‡
‡This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- WARNINGS
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
-
Supplement Facts
Serving Size 1 Softgel (Total daily amount: 2 softgels per day)
Servings Per Container 120
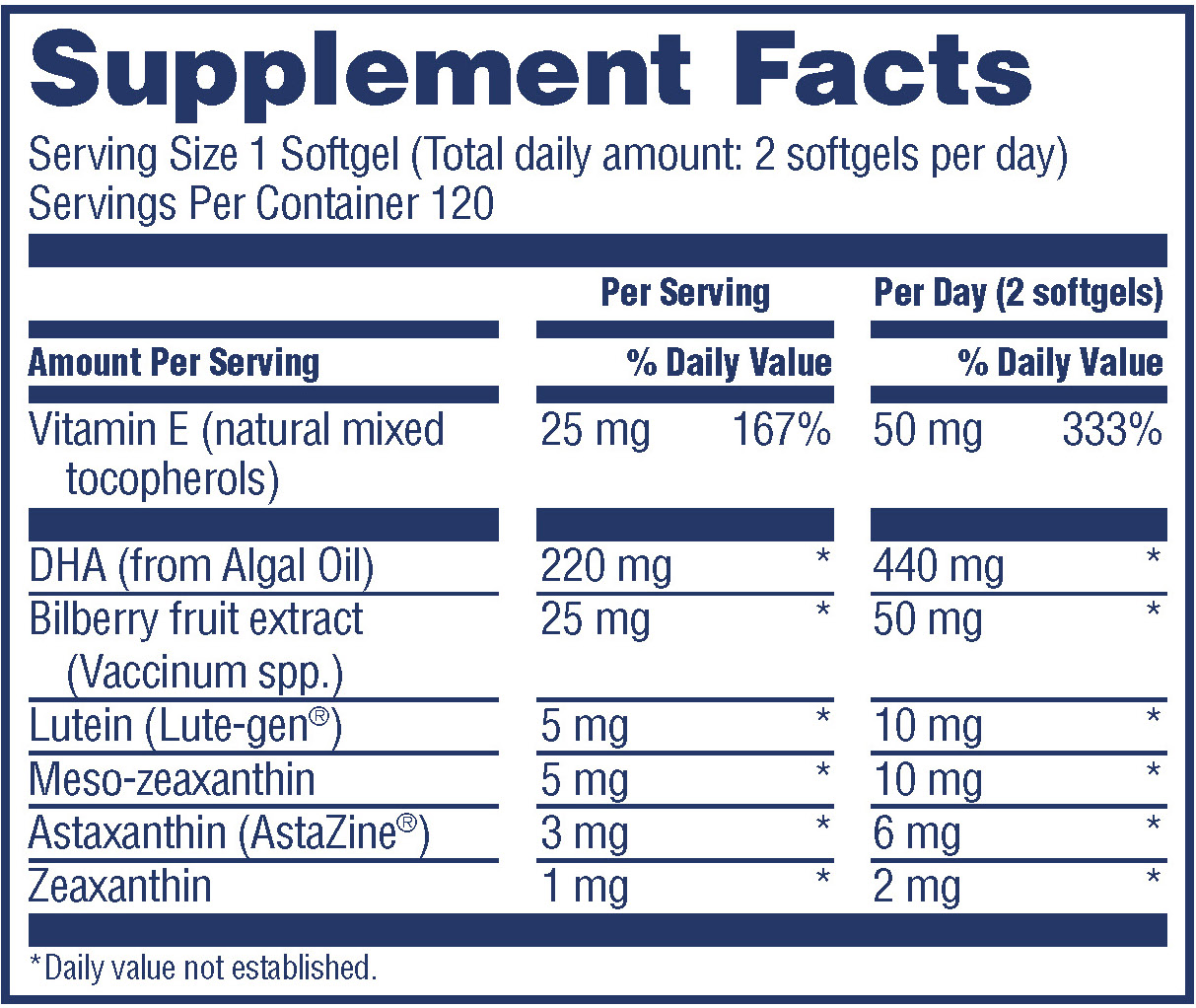
Amount Per Serving Per Serving % Daily Value Per Day (2 softgels) % Daily Value
Vitamin E (natural mixed tocopherols 25 mg 167% 50 mg 333%
DHA (from Algal Oil) 220 mg * 440 mg *
Bilberry fruit extract (Vaccinum spp.) 25 mg * 50 mg *
Lutein (Lute-gen®) 5 mg * 10 mg *
Meso-zeaxanthin 5 mg * 10 mg *
Astaxanthin (AstaZine®) 3 mg * 6 mg *
Zeaxanthin 1 mg * 6 mg **Daily value not established.
- Other ingredients:
- SPL UNCLASSIFIED SECTION
- DOSAGE & ADMINISTRATION
- Vista Advanced Carotenoid Formula 120sgel
-
INGREDIENTS AND APPEARANCE
VISTA ADVANCED CAROTENOID FORMULA
vista advanced carotenoid formula capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77790-007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BILBERRY (UNII: 9P2U39H18W) (BILBERRY - UNII:9P2U39H18W) BILBERRY 25 mg in 908.35 mg .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 25 mg in 908.35 mg ZEAXANTHIN (UNII: CV0IB81ORO) (ZEAXANTHIN - UNII:CV0IB81ORO) ZEAXANTHIN 1 mg in 908.35 mg LUTEIN (UNII: X72A60C9MT) (LUTEIN - UNII:X72A60C9MT) LUTEIN 5 mg in 908.35 mg ASTAXANTHIN (UNII: 8XPW32PR7I) (ASTAXANTHIN - UNII:8XPW32PR7I) ASTAXANTHIN 3 mg in 908.35 mg ZEAXANTHIN, MESO- (UNII: 3O63K300I5) (ZEAXANTHIN, MESO- - UNII:3O63K300I5) ZEAXANTHIN, MESO- 5 mg in 908.35 mg SCHIZOCHYTRIUM DHA OIL (UNII: 2GQR19D8A4) (SCHIZOCHYTRIUM DHA OIL - UNII:2GQR19D8A4) SCHIZOCHYTRIUM DHA OIL 220 mg in 908.35 mg Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) VACCINIUM MYRTILLUS ANTHOCYANOSIDES (UNII: R911H793SU) Product Characteristics Color red Score no score Shape OVAL Size 18mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77790-007-12 120 mg in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2020 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/01/2020 Labeler - Red Wedding LLC (117181523) Establishment Name Address ID/FEI Business Operations Best Formulations, Inc. 147341796 manufacture(77790-007)


