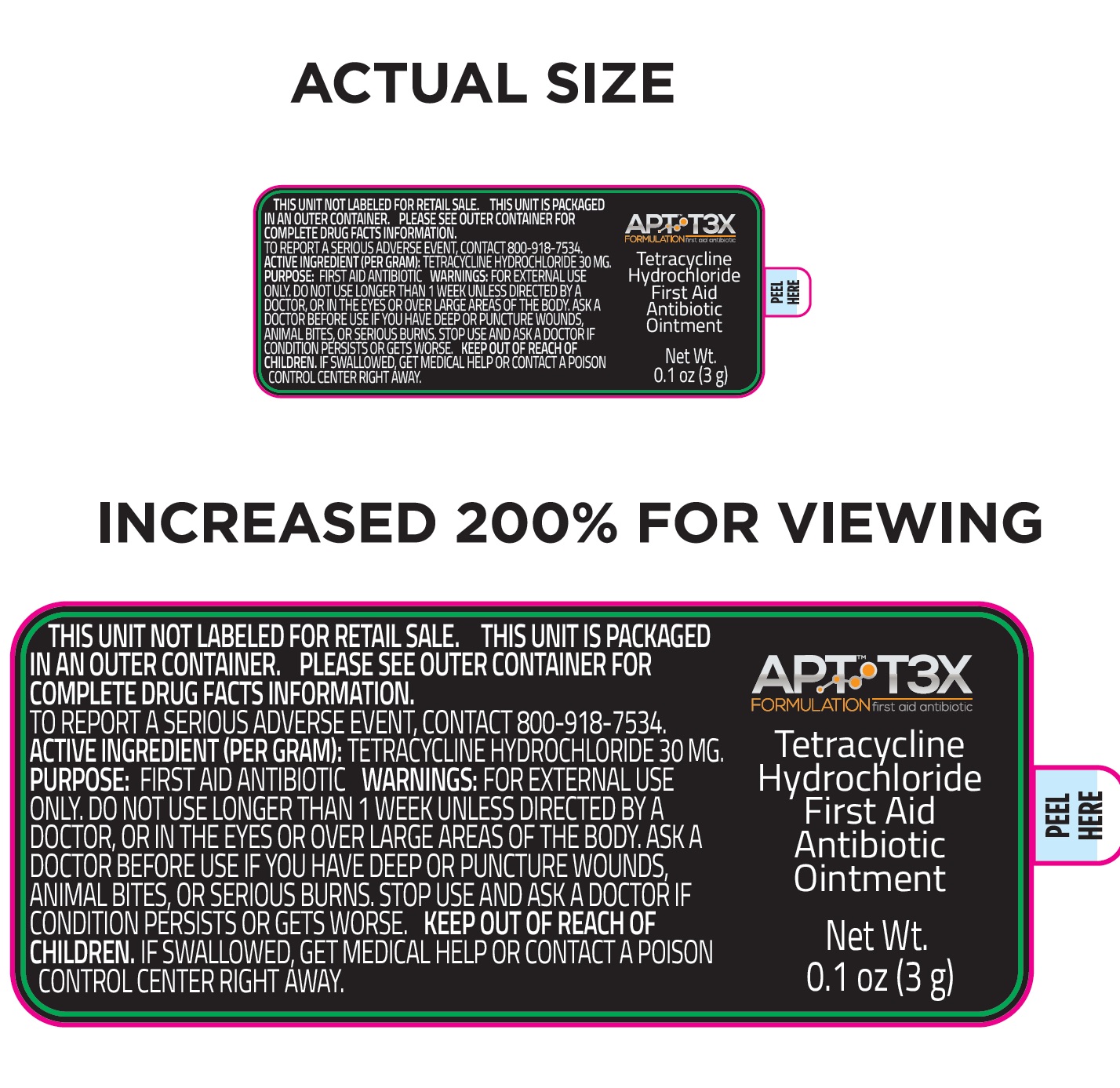
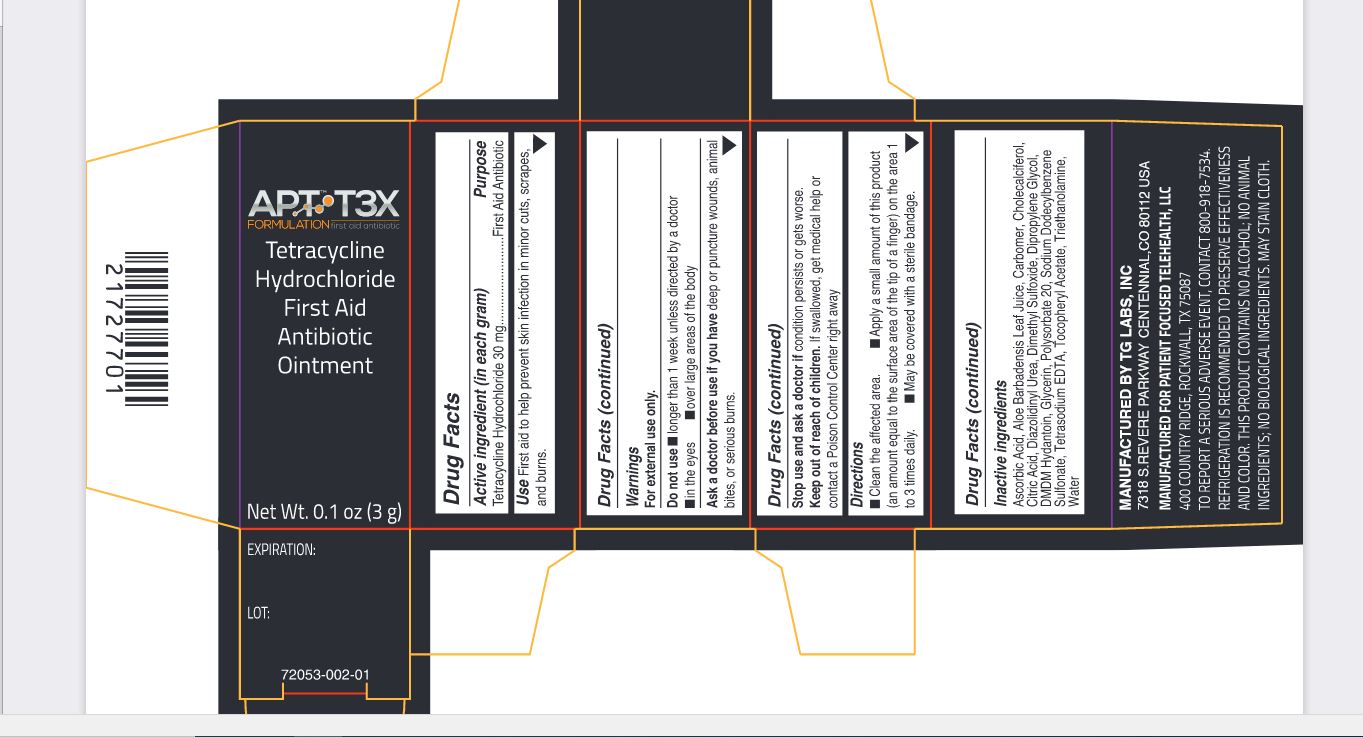
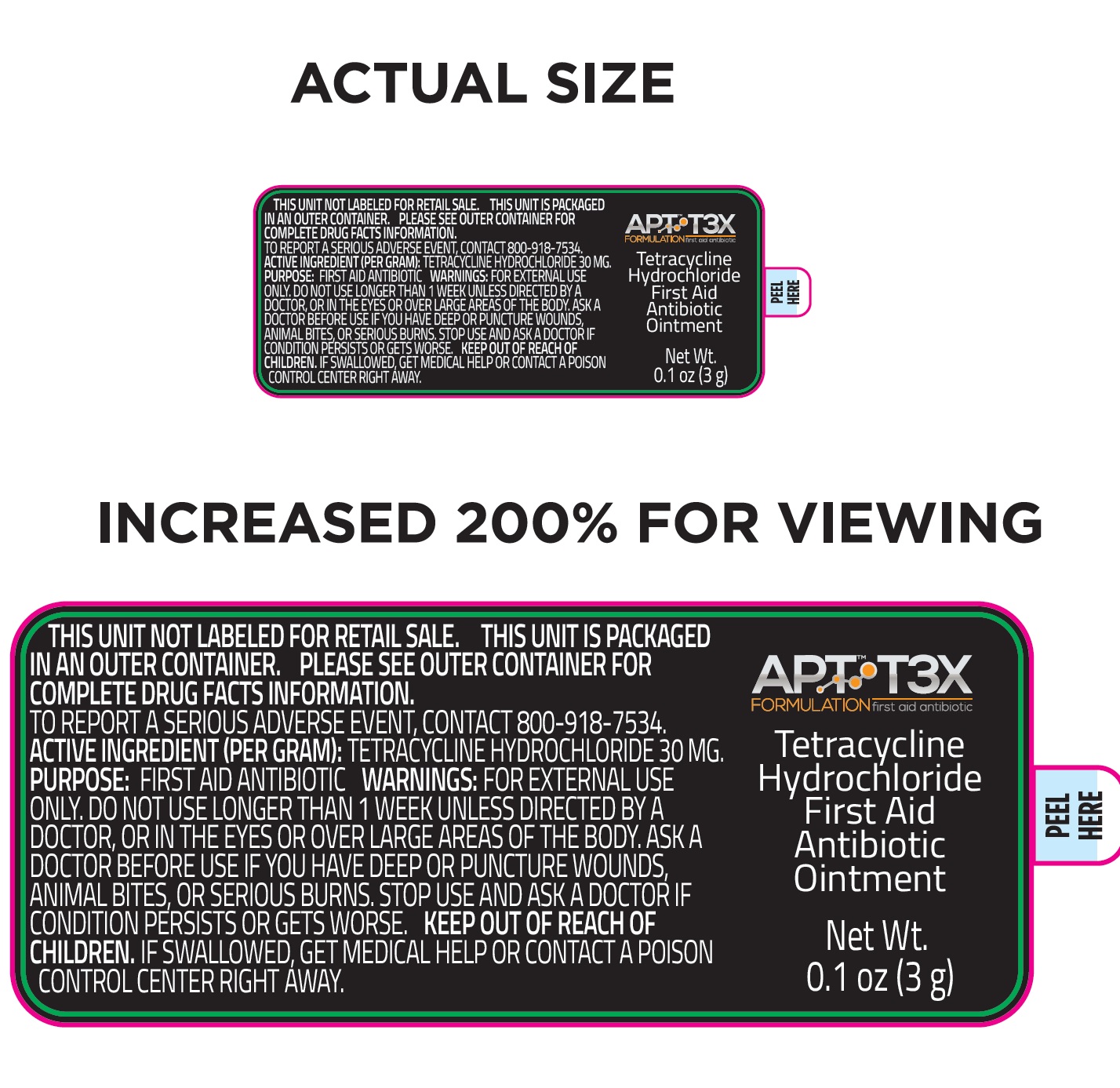
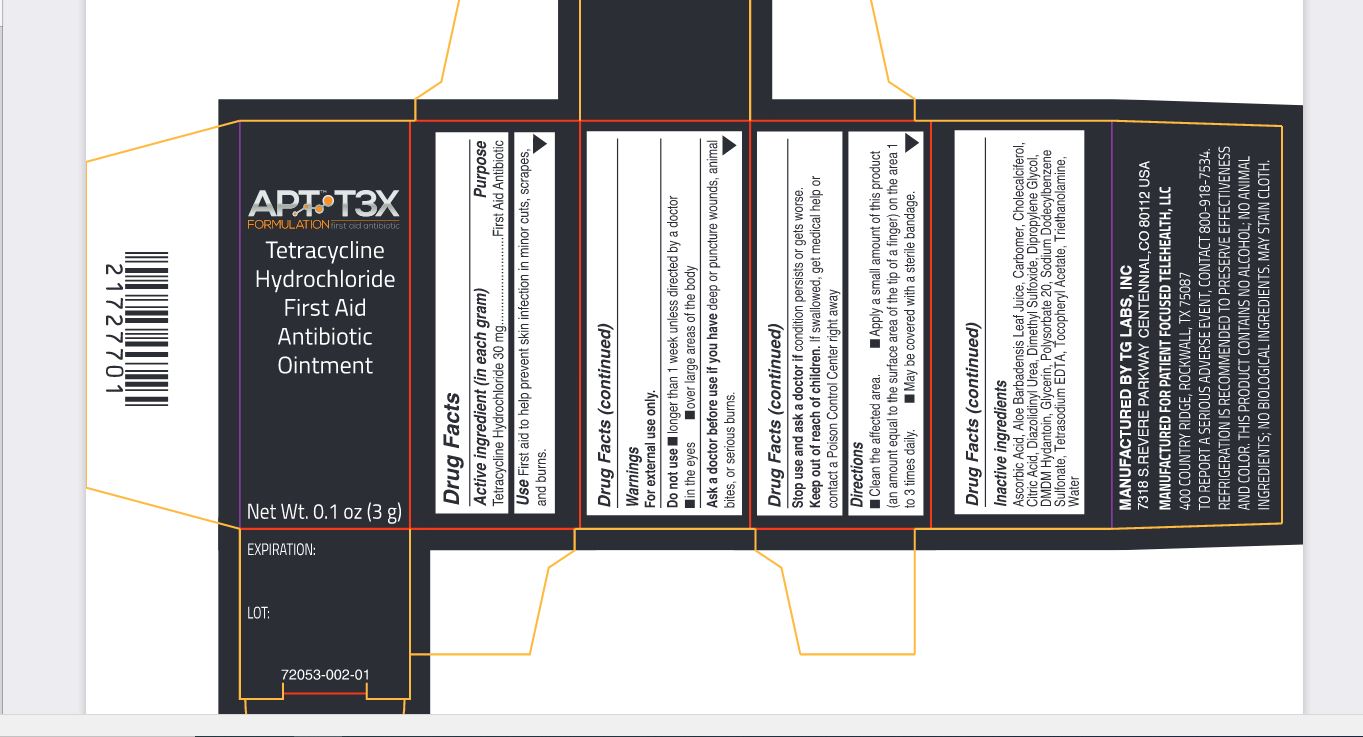
Label: APT T3X TETRACYCLINE HYDROCHLORIDE FIRST AID ANTIBIOTIC- tetracycline hydrochloride ointment
- NDC Code(s): 72053-002-01
- Packager: Patient Focused Tele-Health, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each gram)
- Use
- Warnings
- Directions
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
APT T3X TETRACYCLINE HYDROCHLORIDE FIRST AID ANTIBIOTIC
tetracycline hydrochloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72053-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACYCLINE HYDROCHLORIDE (UNII: P6R62377KV) (TETRACYCLINE - UNII:F8VB5M810T) TETRACYCLINE HYDROCHLORIDE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) CHOLECALCIFEROL (UNII: 1C6V77QF41) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) DIPROPYLENE GLYCOL (UNII: E107L85C40) DMDM HYDANTOIN (UNII: BYR0546TOW) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) EDETATE SODIUM (UNII: MP1J8420LU) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72053-002-01 1 in 1 BOX 09/01/2020 1 3 g in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 09/01/2020 Labeler - Patient Focused Tele-Health, LLC (081008911) Establishment Name Address ID/FEI Business Operations TG Labs, Inc. 831504621 manufacture(72053-002)