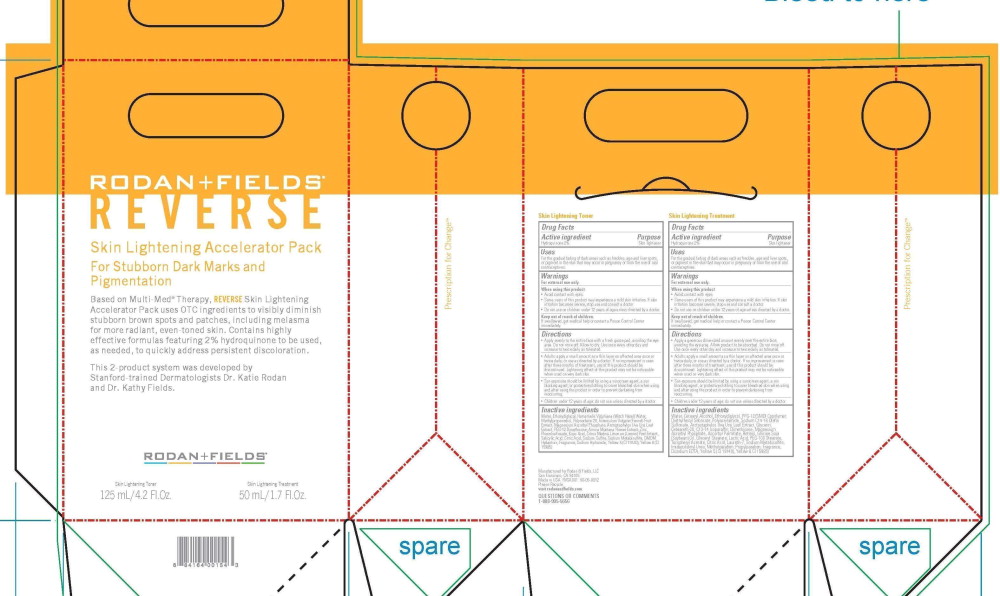
SKIN LIGHTENING ACCELERATOR PACK- hydroquinone
Rodan & Fields, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Skin Lightening Accelerator Pack
Uses
For the gradual fading of dark areas such as freckles, age and liver spots, or pigmnet in the skin that may occur in pregnancy or from the use of oral contraceptives
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Contol Center immediately
Some users of thsi product may experience a mild skin irritation. If skin irritation becomes severs, stope use and consult a doctor
Warnings
For external use only
When using this product
- Avoid contact with eyes
- do no use on children under 12 years of age unless directed by a doctor
Directions -Skin Lightening Treatment
- Apply a genourous dime-sized amount evenly over the entire face, avoiding the eye area. Allow product to be absorbed. Do not rinse off. Use once every other day and increase to twice daily as tolerated.
- Adults: apply a small amount as a thin layer on affected areaonce or twice daily, or use as directed by a doctor. If no improvement is seen after thrre months of treatment, use of this product should be discontinued. Lightening effect of this product may not be noticeable when used on very dark skin.
- Sun exposure should be limited by using a sunscreen agent, a sun blocking agent, or protective clothing to cover bleached skin when using and after using the product in order to prevent darkening from reocurring
- Children under 12 years of age: do not use unless directed by a doctor
Directions -Skin Lightening Toner
- Apply evenly to the entire face with a fresh gauze pad, avoiding the eye area. Do not rinse off. Allow to dry. Use once every other day and increase to twice daily as tolerated.
-
Adults: apply a small amount as a thin layer on affected areaonce or twice daily, or use as directed by a doctor. If no improvement is seen after thrre months of treatment, use of this product should be discontinued. Lightening effect of this product may not be noticeable when used on very dark skin.
-
Sun exposure should be limited by using a sunscreen agent, a sun blocking agent, or protective clothing to cover bleached skin when using and after using the product in order to prevent darkening from reocurringChildren under 12 years of age: do not use unless directed by a doctor
Skin Lightening Treatement
Water, Cetearyl Alcohol, Ethoxydiglycol, PPG-12/SMDI Copolymer, Diethylhexyl Sebacate, Polyacrylamide, Sodium C14-16 Olefin Sulfonate, Arctostaphylos Uva Ursi Leaf Extract, Glycerin, Ceteareth-20, C13-14 Isoparaffin, Dimethicone, Magnesium Ascorbyl Phosphate, Ascorbyl Palmitate, Retinol, Glycine Soja (Soybean) Oil, Glyceryl Stearate, Lactic Acid, PEG-100 Stearate, Tocopheryl Acetate, Citric Acid, Laureth-7, Sodium Metabisulfite, Imidazolidinyl Urea, Methylparaben, Propylparaben, Fragrance, Disodium EDTA, Yellow 5 (CI 19140), Yellow 6 (CI 15985)
Skin Lightening Toner
Water, Ethoxydiglycol, Hamamelis Virginiana (Witch Hazel) Water, Methylpropanediol, Polysorbate 20, Foeniculum Vulgare (Fennel) Fruit Extract, Magnesium Ascorbyl Phosphate, Arctostaphylos Uva Ursi Leaf Extract, PEG-12 Dimethicone, Arnica Montana Flower Extract, Zinc Phenolsulfonate, Kojic Acid, Citrus Medica Limonum (Lemon) Peel Extract, Salicylic Acid, Citric Acid, Sodium Sulfite, Sodijm Metabisulfite, DMDM Hydantoin, Fragrance, Sodium Hydroxide, Yellow 5 (CI 19140), Yellow 6 (CI 15985)
| SKIN LIGHTENING ACCELERATOR PACK
hydroquinone kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Rodan & Fields, LLC. (051659584) |