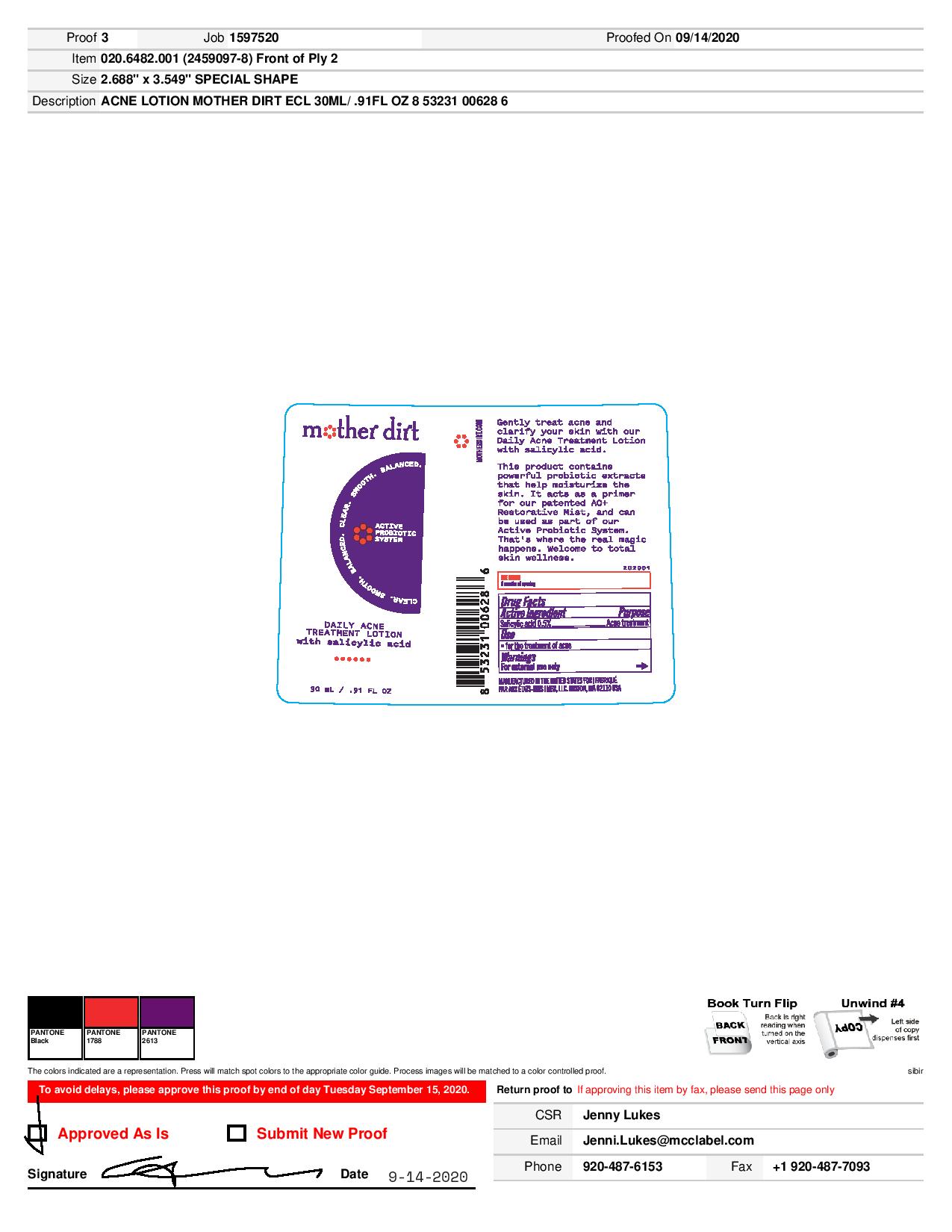
MOTHER DIRT DAILY WITH SALICYLIC ACID- acne lotion
MBX Llc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Mother Dirt Daily Treatment Acne Lotion with Salicylic Acid
Warnings
For external use only
When using this product: skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Directions
Clean the skin thoroughly before applying this product.
Cover the entire affected area with a thin layer one to three times daily.
Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
water, glycerin, ethyl linoleate, astrocaryum tucuma seed butter, glyceryl cocoate, jojoba esters, ethyl olivate, lactobacillus ferment, galactoarabinan, cellulose, glucomannan, glycolipids, lactobacillus, morinda citrifolia extract, chitosan, arginine, cocos nucifera (coconut) fruit extract, stellaria media (chickweed) extract, olea europaea (olive) leaf extract, bacillus ferment
| MOTHER DIRT DAILY
WITH SALICYLIC ACID
acne lotion |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - MBX Llc (116939094) |